Johnson & Johnson completes V-Wave acquisition
Johnson & Johnson has finalized its acquisition of V-Wave an Israeli medical device company focused on developing advanced heart failure technologies. The two companies already have a long history of working together; Johnson & Johnson originally invested in V-Wave back in 2016.
Johnson & Johnson first announced the deal back in August. While the initial purchase price is $600 million, the final total could reach approximately $1.7 billion if certain regulatory and commercial milestones are met.
V-Wave is now officially part of Johnson & Johnson MedTech, joining Shockwave Medical, Abiomed, Biosense Webster, Cerenovus and the other big healthcare technology companies Johnson & Johnson has scooped up in recent years.
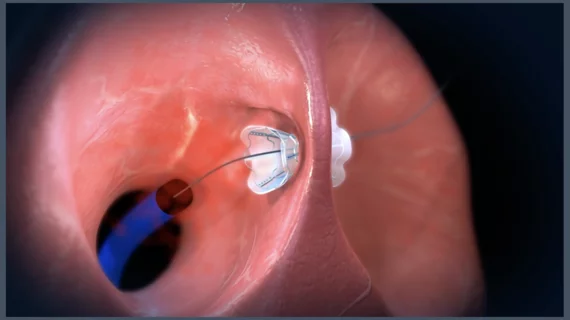
V-Wave’s premier technology is the Ventura Interatrial Shunt System, a small implantable device designed to reduce pressure on the left atrium and the lungs in patients with heart failure with reduced ejection fraction (HFrEF). While the device has not yet gained full approval from the U.S. Food and Drug Administration (FDA), it did receive the FDA’s breakthrough device designation in 2019.
“We’re excited to officially welcome V-Wave to Johnson & Johnson MedTech,” Tim Schmid, executive vice president and worldwide chairman of Johnson & Johnson MedTech, said in a statement. “V-Wave’s novel implantable device, the Ventura Interatrial Shunt, offers tremendous promise for patients experiencing HFrEF. This technology has the potential to be the first device of its kind to market. We look forward to working with the talented V-Wave team to bring this transformative innovation to patients.”
V-Wave’s Ventura device linked to positive patient outcomes
The small Ventura device, which anchors to a patient’s fossa ovalis to prevent migration or embolization, is implanted via an interventional procedure with fluoroscopy and echocardiography guidance.
The Venture device was the focus of the very first late-breaking clinical trial presented at ACC.24, the annual meeting of the American College of Cardiology. Gregg Stone, MD, a professor of cardiology and population health sciences at Icahn School of Medicine at Mount Sinai, presented data from RELIEVE-HF, a randomized trial designed to evaluate the safety and effectiveness of treating heart failure patients with V-Wave’s new technology. RELIEVE-HF included more than 500 patients randomized to either undergo treatment with the device or undergo a placebo procedure.
After a median follow-up period of 22 months, Stone reported that treatment with the device was safe for all patients and provided significant relief for patients presenting with HFrEF. Patients with heart failure with preserved ejection fraction, however, did not benefit from treatment.
Stone spoke with Cardiovascular Business at ACC.24 about the trial, emphasizing that its impact on patients with HFrEF was “very, very positive.”