Medical device company raises another $105M for new-look heart pump
Magenta Medical, an Israeli healthcare technology company, has closed a funding round worth $105 million. The funds are expected to support clinical growth in mechanical circulatory support (MCS) and help secure U.S. Food and Drug Administration (FDA) approval for the company’s Elevate percutaneous left ventricular assist device (LVAD), which it describes as the “world’s smallest heart pump.”
Novo Holdings, the controlling shareholder of international healthcare company Novo Nordisk, led the funding round. Viking Global Investors, RA Capital Management, OrbiMed, New Enterprise Associates, JVC Investment Partners and ALIVE-Israel HealthTech Fund all provided additional investments.
“Magenta is thrilled to add these exceptional MedTech investors to its mission of disrupting the MCS space,” Magenta Medical CEO David Israeli, MD, said in a statement. “Together with our existing partners, we are fortunate to have brought together a world-class group of investors that has both the resources and expertise to shepherd Magenta through regulatory approvals and commercial growth.”
“Magenta's technology stands at the forefront of innovation in the MCS field and has the potential to significantly improve outcomes in patients with severe cardiovascular conditions,” added Eric Snyder, a partner in venture investments with Novo Holdings US. “We look forward to supporting Magenta's team in bringing better care to even more patients in need of mechanical circulatory support.”
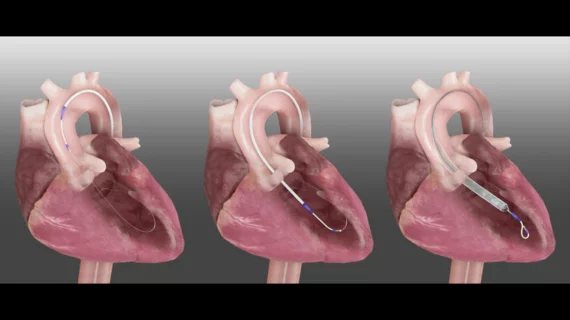
Additional context about Magenta Medical’s Elevate technology
The Elevate device, which previously received the FDA’s breakthrough device designation for both high-risk percutaneous coronary intervention and cardiogenic shock, is folded and then inserted percutaneously through a small puncture in the patient’s groin. The operator delivers the device fully sheathed, through the aorta and across the aortic valve. The device then expands inside the heart, with operators controlling flow through the pump based on the patient’s specific needs.
“Magenta's technology will potentially enable physicians to rely on a single device to treat the full spectrum of MCS indications and is expected to eliminate the need to escalate therapy to a different device and subject patients to unnecessary and invasive replacement procedures,” Israeli said in the same statement.
In May 2023, Magenta Medical closed a financing round worth $55 million. OrbiMed led that particular round, and New Enterprise Associates, Pitango VC and ALIVE – Israel HealthTech Fund also participated.
Abiomed has described its Impella ECP heart pump as the world’s smallest heart pump for years now. The company was also an early investor in Magenta Medical, contributing to a financing round worth $15 million back in 2017, years before it was acquired by Johnson and Johnson.