Implantable atrial shunt therapy trial identifies treatable HFpEF patients
The REDUCE LAP-HF II randomized clinical trial for the Corvia Atrial Shunt in heart failure patients with preserved (HFpEF) or mildly reduced (HFmrEF) ejection fraction had mixed results. While the study identified a responder group for this first-of-its-kind implantable heart failure therapy, placement of an atrial shunt device did not reduce the total rate of heart failure events or improve health status in the overall population of patients.
There were hopes this technology would offer significant relief of heart failure symptoms, but the data did not show this in all patients.
Results were presented at the Technology and Heart Failure Therapeutics (THT) 2022 conference this week and primary results were published online in The Lancet.[1] Publication of the responder group analysis is pending.
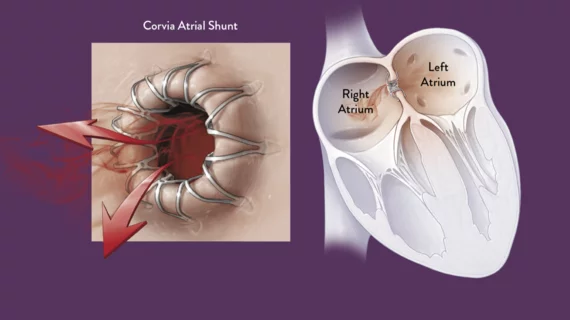
The study is investigating the safety and efficacy of the dime-sized device, which is implanted in a cath lab in the atrial septum to directly address elevated left atrial pressure (LAP), the primary contributor of HF symptoms. The stent-like device maintains a shunt opening between the two atria to reduce the blood pressure.
Corvia said the overall outcome of the trial was neutral, but the data suggests patients with normal exercise pulmonary vascular resistance (PVR) and without a pacemaker represent a responder group to this technology. The study authors said these patients derived significant clinical benefit from the shunt therapy, which makes atrial shunting the first implantable therapy to demonstrate effectiveness in HFpEF.
"In this first-of-its-kind device trial for a complex and heterogenous type of heart failure, we have identified a large potential responder population with meaningful clinical benefit. The ability to predict responders and non-responders is ground-breaking and has significantly advanced our understanding of the role of atrial shunting in HFpEF," co-principal investigator Sanjiv Shah, MD, director of research for the Bluhm Cardiovascular Institute and director of the HFpEF program at Northwestern University Feinberg School of Medicine, said in a statement.
The REDUCE LAP-HF II trial is the world's first phase III trial to evaluate an atrial shunt in heart failure patients to reduce HF symptoms, decrease HF-related hospitalizations and improve quality of life through a reduction in left atrial pressure. The study randomized 626 patients at 89 centers across the U.S., Canada, Europe, Australia and Japan. Patients with normal exercise PVR, indicating the absence of pulmonary vascular disease, and without a pacemaker, derived significant clinical benefit, including a reduction in heart failure events compared to sham (0.12 vs. 0.22 events per patient-year, p=0.007) and a significant and clinically meaningful difference in health status improvement over sham (+5.5 points) as assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score.[2]
"Prior to this study, we knew patients with significant pulmonary vascular disease (PVD) would be very unlikely to benefit from atrial shunt treatment. However, we didn't fully appreciate the critical role that invasive exercise phenotyping may have in uncovering the degree of PVD that allows patients to benefit from atrial shunting," added Barry Borlaug, MD, professor of medicine and director of circulatory failure research at Mayo Clinic. "While further study is needed, with appropriate patient selection, atrial shunting may be a great option for HFpEF patients without any form of PVD. In REDUCE LAP-HF II, treated patients with normal pulmonary vasculature confirmed through exercise, had a significantly greater likelihood of clinical benefit than sham control, with a lower HF event rate and a significant and clinically meaningful KCCQ improvement."
The Corvia Atrial Shunt is a transcatheter device that can be implanted by an interventional cardiologist or electrophysiologist during a minimally invasive, outpatient procedure. The Corvia Atrial Shunt is the most clinically studied atrial shunt for the reduction of LAP in symptomatic HF patients. It was granted Breakthrough Device designation by the FDA in 2019. The Corvia Atrial Shunt is an investigational device and is not available for commercial distribution in the United States. The device is available for sale in the European Union.
Related Intra-atrial Shunt Content:
VIDEO: Overview of intra-atrial shunts to treat heart failure — Interview with Peter Fail, MD
Implantable atrial shunt therapy trial identifies treatable HFpEF patients
Researchers share first human data on new interventional shunt procedure for HFpEF
Corvia Medical raises $54M to continue studying implantable heart failure treatment
Edwards invests in 2 companies to boost transcatheter heart treatment portfolio
4 promising heart failure therapies interventional cardiologists should keep an eye on
References: