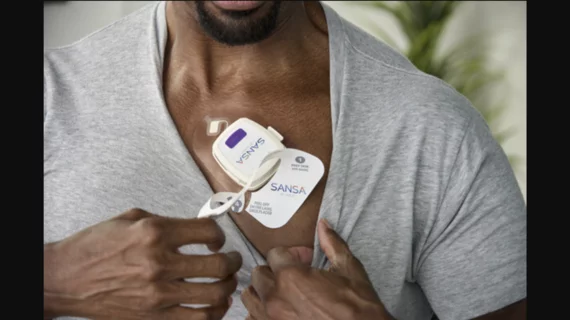
FDA clears chest-worn patch for simultaneous ECG, sleep apnea monitoring
Huxley Medical, an Atlanta-based medical device company, has secured U.S. Food and Drug Administration (FDA) clearance for its new Sansa device, a chest-worn patch designed to help diagnose sleep apnea in addition to tracking patient data with electrocardiograms (ECGs) and a variety of sensors.
Sansa can be used to track an individual’s heart rate, blood oxygen saturation, respiratory health, chest movement, sleep staging, snoring, body position and actigraphy.
“From my prior experience developing programs to engage sleep physicians and cardiologists, I know first-hand the difficulties in getting patients with sleep apnea and comorbid arrhythmias diagnosed and managed,” Chris Hallett, Huxley Medical's co-founder and chief commercial officer, said in a statement. “Sansa will begin to eliminate these barriers for physicians and patients.”
The FDA’s decision was based in part on early data from a new clinical trial exploring the device’s safety and ability to diagnose mild, moderate and severe sleep apnea. According to Huxley Medical, Sansa was associated with “high accuracy, sensitivity and specificity” and helped improve patient compliance.
The patch’s ECG capabilities, and its potential to help treat heart patients, may make it an especially important tool among cardiologists.
“As electrophysiologists, we are already quite comfortable in using patch-based ECG monitors for arrhythmia monitoring,” Suneet Mittal, MD, chair of the cardiovascular service line and director of electrophysiology at Valley Health System, said in the same statement. “The ability to diagnose sleep apnea using the same platform using the Sansa device represents an exciting opportunity to manage two diseases that often co-exist, namely atrial fibrillation and sleep apnea.”
Huxley Medical is privately owned and has raised more than $20 million since being founded in 2019.