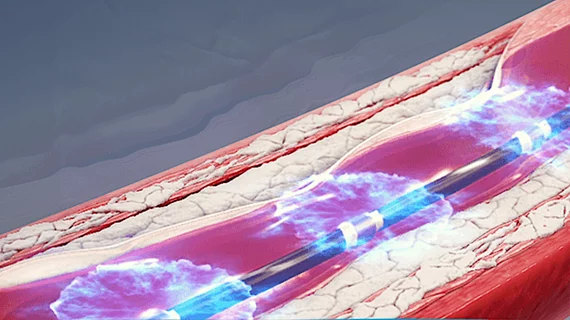
Shockwave’s IVL technology gains FDA approval for treating advanced coronary artery disease
Shockwave Medical announced Tuesday, Feb. 16, that intravascular lithotripsy (IVL) has gained U.S. FDA approval for the treatment of severely calcified coronary artery disease.
Shockwave started developing IVL in 2009. The technology is already approved for treating peripheral arterial disease.
Doug Godshall, Shockwave’s president and CEO, described this latest approval in a statement as “the culmination of years of technical research, rigorous clinical studies and key learnings from our real-world global experience.”
“We are eager for U.S. cardiologists to have access to this technology and experience how a safe, efficient and predictable calcium modification strategy can positively impact their clinical outcomes,” he added.
The Shockwave statement also highlighted recent research published in the Journal of the American College of Cardiology. The authors of that study, first shared with the public in October 2020, found that IVL “safely and effectively facilitates stent delivery and optimizes stent expansion in patients with severely calcified coronary lesions.”
Another analysis focused on IVL treatment was published in Circulation: Cardiovascular Interventions in September 2020.
Related Content on Intravascular Lithotripsy to Break Up Calcified Lesions:
VIDEO: Shockwave Medical demonstrates faster intravascular lithotripsy at ACC22
Better Together? An Integrated Market for Calcium-modification Strategies
IVL Turns Standard Balloon Technology into a Powerful Calcium-disruption Tool
CMS recommends additional payment for using Shockwave’s IVL technology in hospital setting