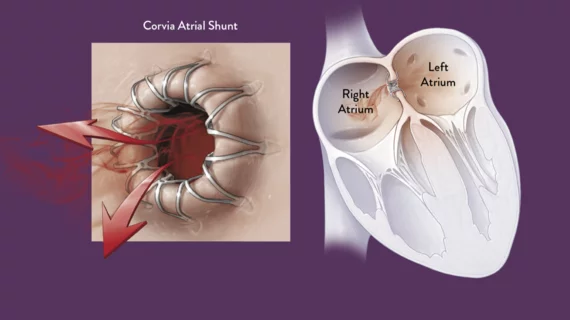
Corvia Medical raises $54M to continue studying implantable heart failure treatment
Corvia Medical has raised $54 million from existing investors to continue researching the safety and effectiveness of its Corvia Atrial Shunt, a transcatheter solution designed to treat heart failure with preserved ejection fraction (HFpEF) and heart failure with mildly reduced ejection fraction (HFmrEF).
“We are grateful to have the ongoing support of our investors as we continue the work to expand access to this novel therapy,” George Fazio, CEO of Corvia Medical, said in a prepared statement. “Corvia is dedicated to bringing atrial shunt therapy to the millions of heart failure patients who might benefit, and this funding allows us to continue advancing toward this important goal.”
“Corvia’s institutional and strategic investors continue to believe in the benefits of atrial shunt therapy and feel strongly that that the Corvia Atrial Shunt has the potential to change the way heart failure patients are treated,” added Paul LaViolette, board chairman of Corvia Medical.
Corvia aims to use these funds to help expand on the REDUCE LAP-HF II clinical trial, which focused on more than 600 heart failure patients treated at 89 different facilities throughout the United States.
Back in February 2022, results from the that trial were presented at THT 2022 and published in The Lancet. The device was associated with a “significant clinical benefit” for HFpEF patients with normal exercise pulmonary vascular resistance and without a pacemaker, though Corvia did indicate that “the overall outcome of the trial was neutral.”
“While further study is needed, with appropriate patient selection, atrial shunting may be a great option for HFpEF patients without any form of PVD,” Barry A. Borlaug, MD, professor of medicine and director of circulatory failure research at Mayo Clinic, said at the time.
The Corvia Atrial Stunt has not yet been fully approved by the FDA, but it was granted the agency’s breakthrough device designation in 2019.
Related Economics Content:
VIDEO: Creating an integrated heart team program in central Texas
Same-day discharge after TAVR is safe for low-risk patients, leads to considerable cost savings
Medtronic, GE Healthcare join forces on new-look continuous monitoring solution
Medical device company raises $23M for fully absorbable vessel closure patches