CMS approves Medicare coverage for transcatheter tricuspid valve replacement
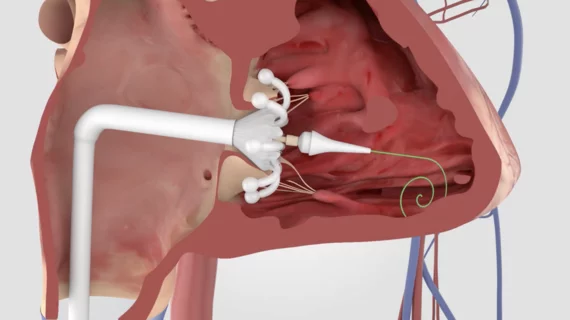
The U.S. Centers for Medicare & Medicaid Services (CMS) has finalized its decision to cover transcatheter tricuspid valve replacement (TTVR) for the treatment of symptomatic tricuspid regurgitation (TR) on a national level.
This only includes TTVR procedures performed on patients who present with symptomatic TR despite already being treated with optimal medical therapy. In addition, CMS noted, the procedure must be viewed as an appropriate treatment option by the patient’s care team, which should include a cardiac surgeon, interventional cardiologist, heart failure specialist, electrophysiologist, multi-modality imaging specialists and an interventional echocardiographer.
“All of the specialists listed above must have experience in the care and treatment of tricuspid regurgitation,” according to CMS.
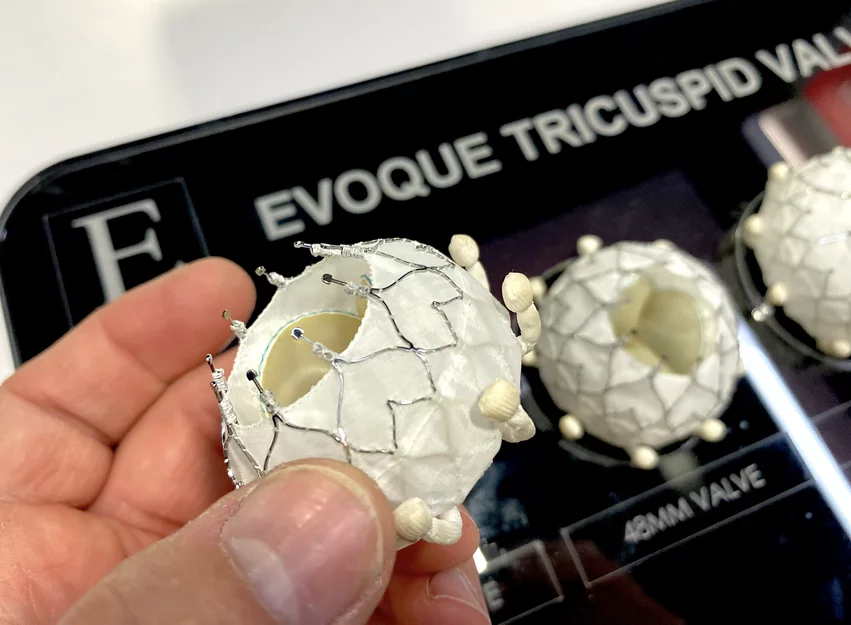
Edwards Lifesciences formally requested that CMS consider a national coverage determination (NCD) in February 2024 after its Evoque system became the first TTVR device to gain approval from the U.S. Food and Drug Administration (FDA) for the treatment of symptomatic TR. The FDA’s decision to approve Evoque was primarily based on positive data from the TRISCEND II clinical trial, which found that treatment was associated with significantly better outcomes than medical therapy alone.
“As a leader in structural heart innovation dedicated to addressing unmet patient needs, Edwards commends CMS for finalizing the first national policy establishing immediate coverage for TTVR for patients suffering from symptomatic TR,” an Edwards spokesperson told Cardiovascular Business. “We believe this policy will enable more patients access to the Evoque TTVR system as a potential treatment option.”
CMS first announced that it was considering a NCD for TTVR in December 2024, requesting public comments through Jan. 18, 2025. The agency is also currently considering a NCD for tricuspid valve repair after a formal request from Abbott. A decision is expected in the weeks ahead.
Click here to read more from CMS on this decision.