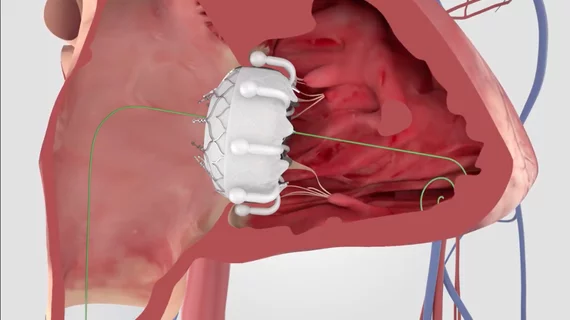
CMS proposes Medicare coverage for transcatheter tricuspid valve replacement
The Centers for Medicare and Medicaid Services (CMS) has proposed offering Medicare coverage on a national level for the treatment of symptomatic tricuspid regurgitation (TR) with transcatheter tricuspid valve replacement (TTVR).
If finalized, coverage would only extend to TTVR procedures performed with a U.S. Food and Drug Administration (FDA)-approved device when patients present with symptomatic TR that does persists after an initial round of treatment with optimal medical therapy. In addition, the patient would be required to be under the care of a multidisciplinary heart team that includes a cardiac surgeon, interventional cardiologist, electrophysiologist, interventional echocardiography and other specialists.
Edwards Lifesciences formally requested CMS to consider a national coverage determination (NCD) back February after its Evoque system became the first TTVR device to gain FDA approval. The FDA’s decision to approve Evoque was primarily based on positive data from the TRISCEND II clinical trial, which found that treatment was associated with significantly better outcomes than medical therapy alone.
“As CMS considers the coverage policy options for TTVR, we urge the agency to consider the evolution of clinical practice using transcatheter therapies,” Daveen Chopra, corporate vice president for transcatheter mitral and tricuspid therapies for Edwards Lifesciences, wrote in the request. “The first transcatheter technology NCD was implemented in 2012 for the coverage of transcatheter aortic valve replacement. The introduction of this novel transcatheter technology, along with the NCD, drove hospital infrastructure adapting to provide transcatheter technologies, clinical practice evolving to improve outcomes, technologies maturing, and this once novel procedure becoming a well-established treatment modality that improves the quality of life of nearly 100,000 U.S. patients each year. Similarly, mitral valve transcatheter technologies have been incorporated into clinical practice, furthering the life of Medicare beneficiaries every day.”
Public comments on this proposal are now being accepted until Jan. 18, 2025. A final decision is expected by March 2025.
CMS previously requested feedback on a proposed NCD to cover tricuspid valve repair after a formal request by Abbott. The open comment period closed in November, and a final decision is expected by April 2025.
Additional details from CMS are available here.