CMS considers Medicare coverage options for tricuspid valve repair at Abbott’s request
The Centers for Medicare and Medicaid Services (CMS) is officially considering whether or not to offer Medicare coverage on a national level for the treatment of symptomatic tricuspid regurgitation (TR) with tricuspid valve transcatheter edge-to-edge repair (T-TEER).
The news comes after Abbott formally requested CMS develop a national coverage determination (NCD) for T-TEER.
“Abbott believes that a national coverage policy for T-TEER will ensure long-term, predictable, and consistent coverage for all Medicare beneficiaries,” Barbara J. Calvert, Abbott’s director of medical products reimbursement, said in the company’s request.
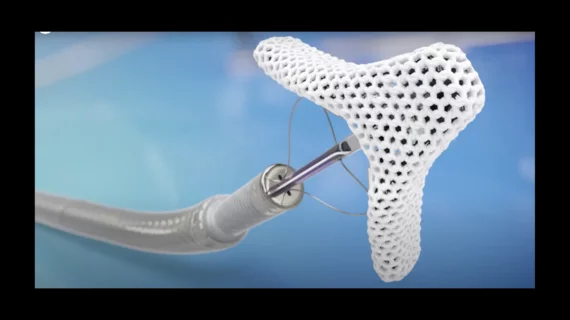
Back in April, Abbott gained approval from the U.S. Food and Drug Administration (FDA) for its T-TEER device, TriClip. TriClip is delivered through a vein in the patient’s leg, repairing the tricuspid valve by clipping together flaps of tissue using the same technology found in Abbott’s popular MitraClip device.
TriClip was the second medical device approved by the FDA for the treatment of TR. The first was the Evoque transcatheter tricuspid valve replacement (TTVR) system from Edwards Lifesciences, which gained approval in February.
In addition, many other devices for T-TEER and TTVR are already in development.
“There are more devices in clinical trials for the tricuspid valve at this time than we could possibly discuss,” Andrew Rassi, MD, a cardiologist with Kaiser Permanente’s San Francisco Medical Center, told Cardiovascular Business in an April interview. “Some are farther along than others and only a few have had successful implants in humans to date. The number of devices being studied speaks to the unmet clinical need that will only be partially answered with the two FDA-approved devices.”
How and when will CMS make its Medicare coverage decision for T-TEER?
CMS is now considering all available clinical evidence related to treating TR with T-TEER.
In its proposal, Abbott pointed to previous NCDs focused on transcatheter aortic valve replacement and mitral valve transcatheter edge-to-edge repair (M-TEER) as recent examples of how the new NCD may look. The company also said that CMS could update the M-TEER NCD so that it covers both the mitral and tricuspid valves.
CMS is accepting comments from the public on this topic until Nov. 2, 2024. Those comments can be shared here. The agency expects to complete its analysis by July 2025, though a proposed decision memo could arrive by April 2025.
Abbott 'encouraged' by progress
"The TriClip device represents an important intervention for patients suffering from TR, offering a safe and effective treatment that can greatly improve quality of life," an Abbott spokesperson told Cardiovascular Business. "We are encouraged by CMS's recognition of the importance of addressing TR and its impact on heart failure, and we look forward to contributing our insights during the comment period to support broader patient access to this vital therapy."