New direct wire pacing device shows potential during PCI and TAVR, first-in-human study finds
A new direct wire unipolar pacing device that removes the need for a temporary venous pacemaker (TVP) during certain interventional procedures may be safe and effective, according to a new first-in-human study published in EuroIntervention.[1] This was a small, single-arm analysis; additional research is still needed to confirm the team’s findings.
The study’s authors noted that the use of TVPs during interventional procedures can lead to significant complications. Direct wire pacing has shown some potential as a way to bypass the need of TVPs, but “technical difficulties” and “potential risks” have kept the technique from picking up too much momentum.
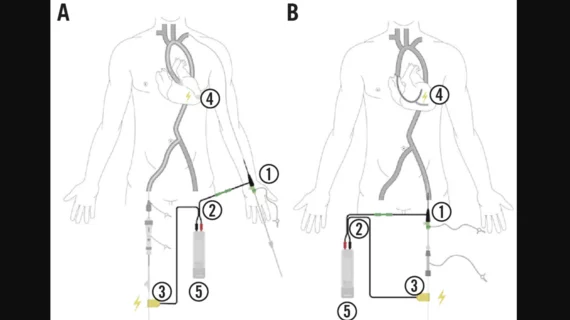
The Electroducer Sleeve, developed by the healthcare technology company Electroducer, was designed to be an alternative option that can help simplify interventional cardiovascular procedures.
In the study, it is described as a “sterile non-implantable device of composed of conductive material that allows the integration of a temporary pacemaker function into the guidewire used during PCI, this providing a simplified technique for direct wire pacing.” The device is connected to an external pacemaker during the procedure. A second cable is then connected to that external pacemaker with a crocodile clip that is also attached to the guidewire.
Can this device lead to better outcomes? To find out, researchers launched a new pilot study focused on 60 patients who underwent an interventional procedure using the device at one of four facilities in France. While 65% of underwent transcatheter aortic valve replacement (TAVR), the remaining patients underwent patients percutaneous coronary intervention (PCI). The mean patient age was approximately 78 years old, and patients were enrolled from July 2020 to January 2021.
Overall, the authors noted, every procedure performed with this new-look device was successful. No major adverse outcomes were reported. Two hematoma were reported over the course of the study; one was linked to the use of Electroducer Sleeve.
“The second hematoma was considered to be unrelated to the device,” wrote first author Jérôme Wintzer-Wehekind, MD, an interventional cardiologist from the Institut Cardiovasculaire de Grenoble in France, and colleagues. “No severe hematomas or bleeding occurred at the device puncture site.”
Looking closer at secondary outcomes, the authors found that two patients presented with radial artery occlusion at a follow-up visit. No femoral artery palpation anomalies were seen in the 28 patients where femoral access was used to insert the device. There were also no allergic or cutaneous adverse reactions.
The study’s primary effectiveness outcome, a hemodynamic effect following each spike delivery, was seen in 90% of patients. However, 12 patients were treated using a pacemaker not recommended for this purpose; among the remaining 48 patients who were treated as recommended, the hemodynamic effect following each spike delivery was seen in 96% of patients.
Another key takeaway from the team’s work was that the Electroducer Sleeve did not appear to lead to longer TAVR procedure times.
“This first-in-human study of the use of a new purpose-built DWP device indicated that the device was safe, effective and well tolerated by the patients,” the authors wrote. “Larger prospective studies are required to confirm these findings and for detailed evaluations of device efficacy.”
Electroducer did help fund this study.
Read the full analysis in EuroIntervention here.