New polymeric heart valve, designed to grow with pediatric patients, implanted for first time
Surgeons at Children’s Hospital of Philadelphia made a bit of history when they completed the world’s first successful implant of a new type of heart valve into a pediatric patient.
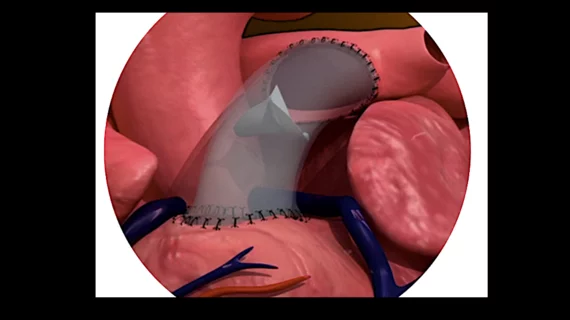
The MASA Valve is a polymeric pulmonary valve designed and manufactured by Pennsylvania-based PECA Labs. It features a proprietary bi-leaflet structural and was designed to grow with the patient, limiting the need for repeat surgeries.
While the MASA Valve is not yet fully approved by the U.S. Food and Drug Administration (FDA), it has received the agency’s humanitarian use device and investigation device exemption. The MASA Valve Early Feasibility Study (MVEFS), designed to help PECA Labs secure FDA approval, is currently enrolling patients in four U.S. sites.
The first-in-human implant involved a 21-month-old patient who had previously received a homograft that became severely stenotic and had to be removed. Early follow-up evaluations confirmed that the patient is recovering as expected with “promising valve functionality.”
“The MASA Valve does not use biologic or foreign tissue, which is expected to reduce the chance of calcification, immunity-based rejection, and valve shrinkage,” David Morales, MD, executive co-director of the Heart Institute at Cincinnati Children’s Hospital and principal investigator of the MVEFS, said in a prepared statement. “The valve design also aims to reduce the amount of anti-thrombogenic drugs administered to the patient over the course of their lifetime. These factors should help greatly improve patient outcomes and could lead to fewer valve replacements over a child’s lifetime. If the study is successful, the MASA Valve could offer a significant improvement in the treatment of cardiovascular congenital heart defects in children and save the patients and the health system significant amounts of money spent on cardiac care.”
“In designing the MASA Valve, we aimed to use our polymer platform to combine the best characteristics of homograft tissue, which reduces the antithrombotic therapy required, and mechanical valves, which offer improved durability, supply availability, and resistance to both calcification and shrinkage,” added Doug Bernstein, CEO of PECA Labs. “We then improved upon them with a novel bi-leaflet design.”