The primary reason Boston Scientific’s TAVR valve fell short in clinical trial
Addressing underexpansion issues could have enabled the Acurate neo2 aortic valve system to perform comparably to leading transcatheter aortic valve replacement (TAVR) systems, according to a new analysis of the ACURATE IDE trial.
The trial, co-led by Michael Reardon, MD, professor of cardiothoracic surgery and the Allison Family Distinguished Chair in Cardiovascular Research at DeBakey Heart and Vascular Center, Houston Methodist Hospital, originally concluded that the Acurate neo2 was inferior to Sapien and Evolut valves. However, post-trial findings have shed new light on those results.
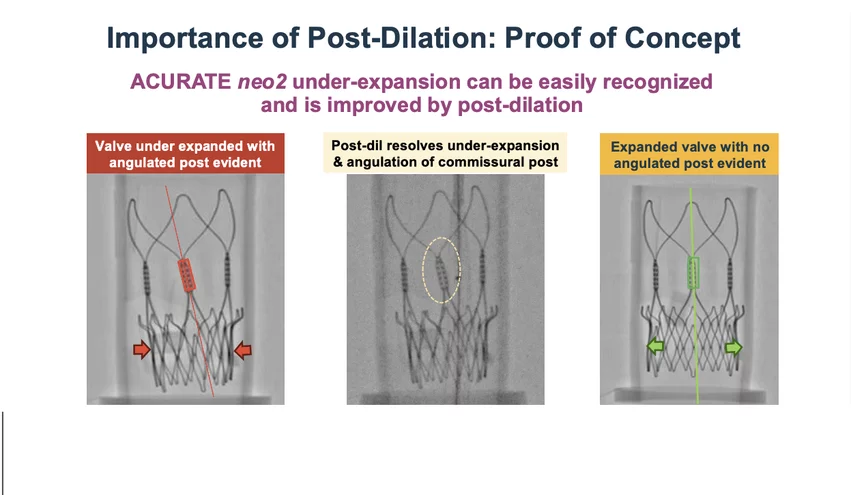
The one-year outcomes presented as a late-breaking trial at the 2024 Transcatheter Cardiovascular Therapeutics (TCT) meeting showed the Boston Scientific TAVR valve was inferior to Sapien and Evolution because it did not meet its primary end-points for death and stroke. Reardon said a detailed review of the data revealed that approximately 20% of the Acurate neo2 valves implanted in the trial were underexpanded, which is associated with significantly worse patient outcomes.
“If you were not fully expanded, it doubled your risk of dying and tripled your risk of having a stroke,” Reardon explained during an interview with Cardiovascular Business.
When the patients with the underexpanded valves were removed from the data, the Acurate neo2 actually performed pretty well. "If I take the death or stroke and look at the one year outcome, there was absolutely no difference in the expanded valves between control," Reardon said.
The trial’s timeline also coincided with the COVID-19 pandemic, which posed significant challenges. Research was halted during the early stages of the pandemic, and once resumed, the frequency of Acurate neo2 implants was significantly reduced, which impacted the level of operator experience using the new valve.
“The average time between Acurate implants at sites was three months,” Reardon noted. “Only 10% of operators implanted 10 or more valves, which limited their familiarity with the device.”
Retrospective insights to identify underexpanded Acurate neo2 valves
Engineers blind to patient outcomes of the trial examined the imaging of the valves for signs of underexpansion. They looked for tell-tale signs of underexpansion, including angulation of the posts and a straight valve waist at the base of the valve, rather than being flared out when the valve is fully expanded in the annulus. Reardon said their findings were pivotal; valves with proper expansion demonstrated no difference in death or stroke rates compared to control valves, while underexpanded valves showed alarming risks.
The key issue, according to Reardon, was that operators were not trained to identify underexpansion during procedures. He said there are similar issues with the Sapien and Evolution, but operators learned how to adopt through experience.
"I do think that now that we see this balloon expansion is going to be an easy way to mitigate that. And based on what we've seen so far, I think if we do expand them out adequately, we'll see outcomes are just as good as control valves," Reardon said.
Next steps for the Acurate neo2
The findings came too late to impact the ACURATE IDE trial, but Reardon believes the lessons learned could inform future use of the Acurate neo2. The valve, widely used in more than 60 countries, boasts attributes like low pacemaker rates and good hemodynamics that make it a strong contender in the U.S. market. Trial data from overseas also continue to show very good outcomes.
He said discussions are ongoing between the device manufacturer and the FDA to determine next steps.
“Now that we recognize under-expansion, this is a correctable issue,” Reardon said. “I’m confident that with proper procedural adjustments, the Acurate neo2 can match the performance of current leading valves.”