FDA grants new valve-in-valve TAVR offering its breakthrough device designation
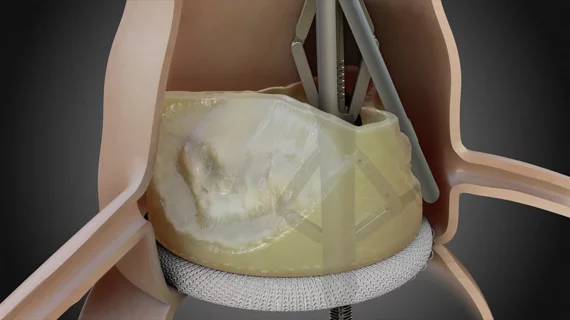
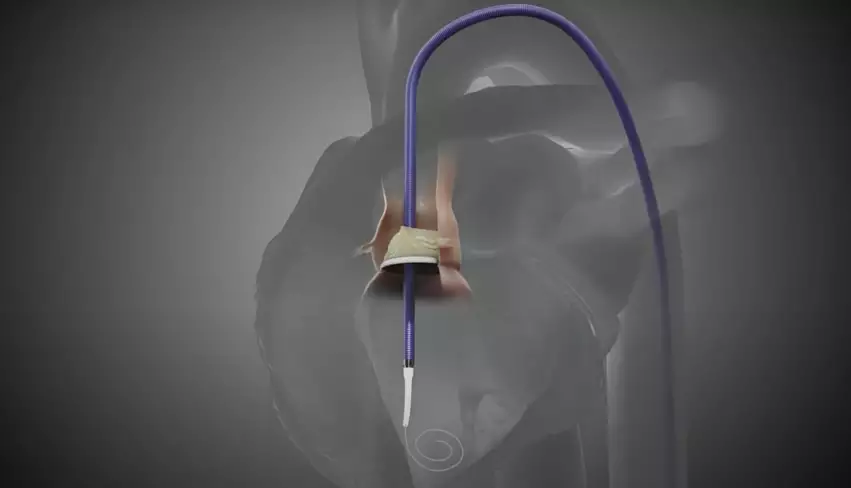
Pi-Cardia, an Israel-based healthcare technology company, has received the FDA’s breakthrough device designation for its ShortCut device designed to help clinicians perform valve-in-valve transcatheter aortic valve replacement (TAVR) on high-risk patients.
According to Pi-Cardia, ShortCut is the first medical device of its kind. Interventional cardiologists use it to split valve leaflets in patients who have been recommended for valve-in-valve TAVR and face a heightened coronary obstruction risk.
“Having been part of Pi-Cardia’s rigorous clinical program, I am thrilled to see the recognition in the importance of ShortCut,” Philippe Genereux, MD, an interventional cardiologist with Morristown Medical Center in New Jersey, said in a statement. “Lifetime management of aortic stenosis calls for leaflet modification solutions like ShortCut to ensure that we are carefully addressing the risk of coronary obstruction before implanting a valve. From what we have seen regarding the ability to easily teach and perform the procedure, ShortCut could be easily adopted by every TAVR center as a critical step pre-implantation, so that patients at risk of coronary obstruction will be safely treated, without disruption of TAVR work-flow.”
The first U.S. patient was treated with ShortCut in December 2022. Genereux was one of the cardiologists involved with that initial procedure. At the time, Pi-Cardia anticipated that the device could gain full FDA approval by as early as 2024.
The FDA’s breakthrough devices program is designed to help medical devices make it through the approval process faster than they would otherwise. The agency’s representatives work directly with the device manufacturer, for example, and any submissions related to the device will be prioritized.
“We are excited to receive this important recognition by the FDA,” Erez Golan, Pi-Cardia's CEO, said in the same statement. “Breakthrough device designation is only awarded to technologies that have the potential to provide more effective treatment or diagnosis for life-threatening or irreversible debilitating diseases or conditions, and it may help accelerate our review process with the FDA this year and bring ShortCut to market for the benefit of patients.”