Edwards makes history, receives FDA approval for transcatheter tricuspid valve replacement device
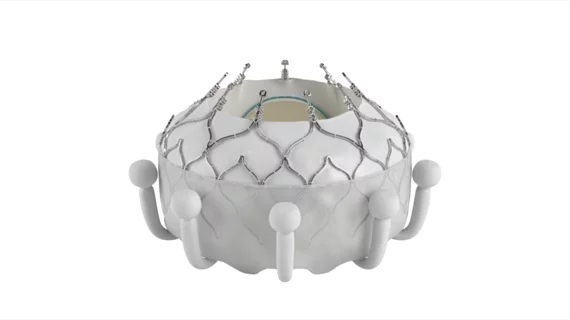
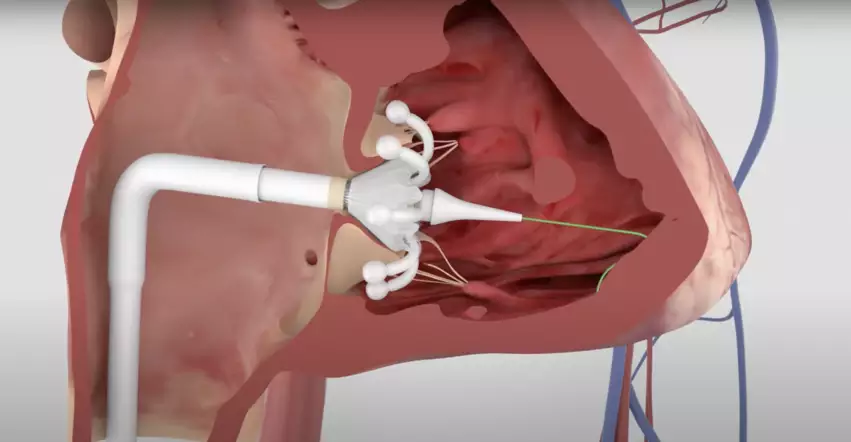
Edwards Lifesciences has received U.S. Food and Drug Administration (FDA) approval for its Evoque transcatheter tricuspid valve replacement (TTVR) system for patients with tricuspid regurgitation (TR). This represents a significant milestone for the structural heart disease community, as Evoque is officially the first TTVR device to gain FDA approval.
“Edwards has a long history of leading innovation and pioneering new therapies to address the unmet needs of patients with structural heart disease,” Daveen Chopra, corporate vice president of transcatheter mitral and tricuspid therapies for Edwards Lifesciences, said in a statement. “We are grateful for the strong collaboration with clinicians all over the world who contributed to the Evoque system now being available through FDA’s breakthrough pathway to provide a treatment option to the many patients in the U.S. suffering with tricuspid valve disease.”
The FDA cleared the Evoque transcatheter tricuspid valve system for patients presenting with symptomatic severe TR that does not improve with optimal medical therapy. Features include a nitinol self-expanding frame, an intra-annular sealing skirt and leaflets made of bovine pericardial tissue.
The device, which already received European CE mark approval last October, will be available in three different sizes once it hits the U.S. market.
The FDA’s approval was primarily based on positive data from the TRISCEND II clinical trial, which found that treatment with Evoque was associated with significantly better outcomes than medical therapy alone.
Early results from TRISCEND II were first shared at TCT 2023 in San Francisco by Susheel Kodali, MD, director of the Structural Heart and Valve Center at Columbia University Irving Medical Center/New York-Presbyterian Hospital and the study’s principal investigator, just days after the device received CE mark approval.
“Patients suffering with TR endure life-impairing symptoms and, until today, had no approved transcatheter treatment options,” Kodali said in a statement. “The Evoque system is able to replace the native tricuspid valve, virtually eliminating TR in a wide range of patients. We see significant improvements in patients’ symptoms and quality-of-life (QOL), including not feeling short of breath and being able to care for themselves, which ranked highest on a patient preference survey conducted at baseline with TRISCEND II pivotal trial patients.”
When Kodali presented those initial TRISCEND II results at TCT 2023, it became one of the most popular topics at the conference. During a press conference, for example, interventional cardiologist Yousif Ahmad, MD, PD, described the trial as a “landmark study.”
“The landscape for tricuspid valve interventions is changing,” he said at the time. “If you look at just at 12 months or two years ago, we had limited options. Now, with the TRILUMINATE data presented last year and this new TRISCEND II data, we could potentially have two randomized controlled trial-proven therapies to offer these patients. It’s very encouraging for the field.”
Additional data from TRISCEND II is expected to be shared at TCT 2024.