More good news for Edwards: ‘Landmark study’ confirms new TTVR system reduces tricuspid regurgitation, improves symptoms
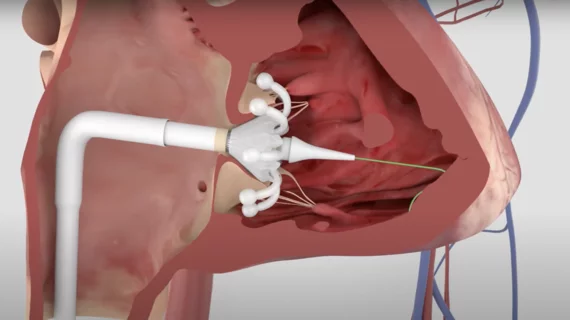
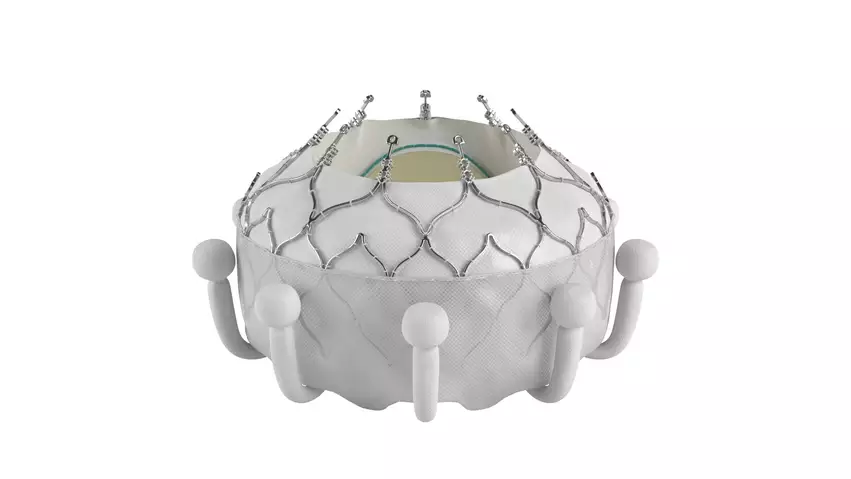
Just days after Edwards Lifesciences received CE mark approval for its Evoque transcatheter tricuspid valve replacement (TTVR) system for tricuspid regurgitation (TR), the company scored another key victory with the device. Highly anticipated data presented at the Transcatheter Cardiovascular Therapeutics (TCT) 2023 meeting in San Francisco showed it is associated with significant reductions in TR and consistent quality of life (QOL) improvements.
Susheel Kodali, MD, an interventional cardiologist with the Columbia University Department of Medicine, opened the late-breaking clinical trials at TCT on Thursday, Oct. 27, by sharing data from the first 150 patients enrolled in the TRISCEND II study. TRISCEND II was designed to evaluate the safety and effectiveness of the combination of the Evoque system and optimal medical therapy (OMT) compared to just OMT alone in patients presenting with severe TR.
Overall, after six months, treatment with the Evoque device was associated with almost completely eliminating TR in nearly 78% of patients. Nearly 99% of patients had moderate or less TR after treatment, and nearly 94% had mild or less TR. TTVR was also associated with clear improvements in QOL as measured by changes in Kansas City Cardiomyopathy Questionnaire and six-minute walk distance results.
Data from additional patients is expected to be shared in the near future. Kodali said drawing from a larger patient population could go a long way toward clarifying the Evoque device’s clinical impact. Patients will ultimately be followed for up to five years.
Edwards could be just months away from a full FDA approval
The Evoque system received the U.S. Food and Drug Administration’s breakthrough device designation back in 2019. Now, Edwards hopes these early TRISCEND II results can help pave the way for a full FDA approval. The agency is expected to make a final decision on approval by the middle of 2024; an FDA advisory committee could meet to make an initial ruling as early as January 2024.
“Patients suffering from severe TR endure debilitating symptoms and poor quality of life and are desperate for effective treatment, as evidenced by the rapid enrollment in this trial,” Daveen Chopra, corporate vice president of transcatheter mitral and tricuspid therapies at Edwards, said in a statement. “These data build upon the promising foundation of results previously published from the single-arm TRISCEND study and add to our growing body of contemporary clinical evidence that enables more patients in need to receive treatment.”
The interventional cardiology community responds to TRISCEND II
Yousif Ahmad, MD, PhD, an interventional cardiologist with Yale School of Medicine, called TRISCEND II a “landmark study” during a TCT press conference.
“The landscape for tricuspid valve interventions is changing,” he said. “If you look at just at 12 months or two years ago, we had limited options. Now, with the TRILUMINATE data presented last year and this new TRISCEND II data, we could potentially have two randomized controlled trial-proven therapies to offer these patients. It’s very encouraging for the field.”
“This is an extremely important arena,” added Wayne Batchelor, MD, director of interventional cardiology research and education with the Inova Health System. “We have to get better safety and efficacy data, but I’m very encouraged by the trials that are ongoing. Only about 500-600 isolated tricuspid valve repair surgeries are done in the United States [per year], but we have a million and a half patients with significant tricuspid disease, so this is clearly an unmet need. I think all of us—physicians, patients and healthcare providers—are looking forward to more results coming out in the next year.”
Does this data tell us enough?
One hot topic of discussion at TCT was the fact that the early data focused so much on QOL metrics as opposed to measurable clinical improvements.
Kodali acknowledged this limitation, but he also defended the usefulness of tracking measurements of QOL.
“Quality of life, from a patient’s perspective, is an important outcome,” he said. “We’re going to have to look at the totality of evidence, right? Are all of the individual endpoints trending in the right direction? Are mortality and heart failure potentially trending, even if we don’t meet a level of significance due to the trial’s number of patients? Are the echocardiographic parameters—RV remodeling, decrease in RV size—trending? We’re going to end up looking at the totality of evidence. This device, unlike TEER, eliminates TR. Whether that elimination of TR will translate to mortality is what we need to see at one year, and I hope it does. Even if it does not, though, QOL is important and a relevant endpoint.”
Roxana Mehran, MD, a professor of medicine and director of interventional cardiovascular research and clinical trials at the Zena and Michael A. Wiener Cardiovascular Institute at Mount Sinai School of Medicine, was complimentary of the research, but did highlight the importance of identifying additional clinical benefits of the device beyond improvements in QOL.
“I personally believe that we absolutely need to show at least some evidence of an improved clinical outcome like hospitalization or heart failure,” she said. “QOL is incredibly important … but I still believe it has to be in the context of clinical outcomes.”