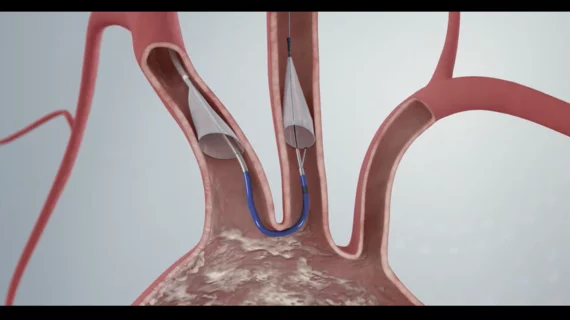
Cerebral protection devices during valve-in-valve TAVR: Cleveland Clinic cardiologists identify key benefits
The use of a cerebral protection device (CPD) during valve-in-valve transcatheter aortic valve replacement (TAVR) procedures is associated with multiple clinical benefits, according to new findings published in the American Journal of Cardiology.[1]
Prior research on the impact of embolic protection devices during TAVR has led to mixed results. When results from the PROTECTED TAVR study were published in the New England Journal of Medicine, for example, they showed that the Sentinel CPD from Boston Scientific failed to reduce a patient’s risk of stroke, but it did decrease the risk of a disabling stroke.
This latest study focused on data from 19,000 U.S. patients who underwent valve-in-valve TAVR from 2017 to 2020. All data came from the Nationwide Readmissions Database, and patients were excluded if they underwent concomitant surgical aortic valve replacement, coronary bypass surgery or thoracic aorta surgery.
Overall, 8.4% of valve-in-valve TAVR patients were treated with a Sentinel CPD. CPD patients had a slightly younger mean age (74.6 vs. 75.9 years old) and were less likely to present with diabetes, atrial fibrillation, carotid artery disease or dialysis-dependent end-stage renal disease.
Patients treated with a CPD were associated with a lower overall stroke rate (2% vs. 4.3%), lower major stroke rate (1.7% vs. 3.3%), shorter median length of stay (2 vs. 3 days) and higher odds of a routine discharge (70.8% vs. 64.1%) than patients treated with no CPD. In addition, CPD use was independently associated with a reduced risk of hospital readmission after 30 days, but not after 180 days.
In-hospital mortality, meanwhile, was comparable between the two treatment groups.
“As stroke is an unpredictable complication of the TAVR procedure, our study is timely as it identifies a patient population that would benefit from CPD use,” wrote corresponding author Samir Kapadia, MD, an interventional cardiologist and chair of the department of cardiovascular medicine at Cleveland Clinic, and colleagues. “Based on our findings, operators may consider utilizing CPD for patients undergoing valve-in-valve TAVR. Data from future trials and registries are required to further substantiate our findings.”
Kapadia has been closely involved in studying the clinical impact of CPD use during TAVR procedures for many years now. He presented initial PROTECTED TAVR findings at TCT 2022, for example, and spoke to Cardiovascular Business about the topic in February 2023.
While this study examined the potential impact of Boston Scientific’s Sentinel device, that company did not fund or influence this work in any way.
Click here to read the full analysis.
Additional context on using embolic protection devices during TAVR
Embolic protection devices work by capturing debris during the aortic valve replacement procedure. The Sentinel device captured debris during 99% of TAVR cases included in PROTECTED TAVR, emphasizing its effectiveness, but the clinical impact of these devices—and the high costs associated with using them—remain hot topics among interventional cardiologists.
When Kapadia spoke to Cardiovascular Business about these devices, he noted that using a Sentinel device adds approximately $3,000 to the overall costs of performing a TAVR procedure. Reimbursement policies have not been updated to include the use of a CPD, meaning these costs will remain a key detail for care teams to consider.
“We are using the hospital's money to purchase the device, so if the evidence does not exist to conclusively use it, no one is going to blame us for not using it,” Kapadia said back in 2023. “As a physician, the question for me is not about the blame, but what is the right thing to do.”
When Kapadia presented the initial PROTECTED TAVR data at TCT, a group of panelists was asked if they would want embolic protection if they or a loved one was undergoing TAVR; all but one panelist said, yes, they would want such a device to be used in such a scenario.