FDA grants AI software for imaging-based heart assessments its breakthrough device designation
Cleerly, a Denver-based healthcare technology company focused on using artificial intelligence (AI) to diagnose heart disease, has received the FDA’s breakthrough device designation for new software that delivers AI-powered coronary artery disease (CAD) risk assessments based on medical imaging results.
The breakthrough devices program is designed to help medical devices make it through the approval process faster than they would otherwise. The agency’s representatives work directly with the device manufacturer, for example, and any submissions related to the device are prioritized.
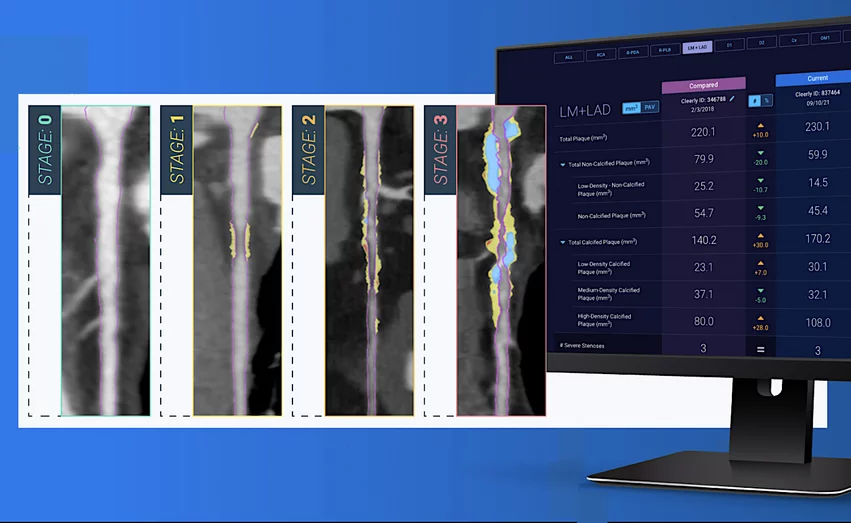
Cleerly’s CAD Staging System scans images for potential signs of coronary atherosclerosis, stenosis and ischemia, helping cardiologists and other physicians manage patients who may be at risk of a major adverse cardiovascular event.
As a result of this approval, CAD Staging System is being added to the FDA’s Total Product Life Cycle Advisory Program (TAP) Pilot, a new program designed to improve patient access to “high-quality, safe, effective and innovative medical devices for years to come.” A total of 36 devices are currently a part of TAP Pilot.
“As Cleerly continues to develop cardiovascular innovations to support patients and healthcare professionals, we are honored to have received breakthrough device designation and join the TAP Pilot,” James K. Min, MD, founder and CEO of Cleerly, said in a statement. “This designation from the FDA highlights the critical need for better heart disease risk assessment methods. Our approach to heart disease, inspired by the most successful preventive care paradigms in medicine—including mammograms, colonoscopies, and lung CTs—enables personalized diagnosis and risk assessment and exemplifies our commitment to enhancing CAD evaluation, aiming to prevent heart attacks before they occur.”
“As our Cleerly CAD Staging System becomes available to physicians and patients, it will provide the rationale for preventive tailored treatment of CAD with risk-based therapy goals,” added Udo Hoffmann, MD, MPH, chief scientific officer of Cleerly.
Cleerly announced that its CAD Staging System will also be validated as part of the TRANSFORM randomized trial first announced back in November 2023. The goal is to enroll up to 7,500 patients for TRANSFORM, including participants with pre-diabetes, type 2 diabetes and metabolic syndrome.