FDA grants anticoagulant its orphan drug designation for treating patients with implanted devices
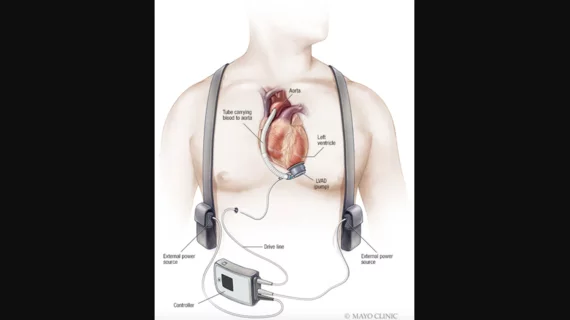
Tecarfarin, a new anticoagulant from Cadrenal Therapeutics, has received the FDA’s orphan drug designation (ODD) for the prevention of thromboembolism and thrombosis in patients with certain implanted devices. The agency’s ruling covers patients with left ventricular assist devices, right ventricular assist devices, biventricular assist devices and total artificial hearts.
Tecarfarin is a vitamin K antagonist originally designed to be used by patients with end-stage kidney disease and atrial fibrillation; it received the FDA’s ODD and fast track designation for this indication. The company then announced it was expanding the drug’s focus to include patients with implanted medical devices in August 2023.
“This second orphan drug designation highlights the expanded need for tecarfarin where existing anticoagulation therapies are inadequate,” Quang Pham, founder, chairman and CEO of Cadrenal Therapeutics, said in a prepared statement. “We are dedicated to advancing tecarfarin through clinical development options as swiftly as possible.”
Cadrenal Therapeutics believes concentrating on patients with these devices can help address an unmet need in cardiology. While all patients with ventricular assist devices require anticoagulation, no direct oral anticoagulants on the market have been specifically approved for this purpose.
The company’s next goal for tecarfarin is receiving an additional ODD from the FDA for providing anticoagulation therapy for patients presenting with thrombotic antiphospholipid syndrome.
Tecarfarin has not yet been approved by the FDA.