Medical device company raises $36M for emitter-free IVL platform
Amplitude Vascular Systems (AVS), a Boston-based medical device company focused on developing new intravascular lithotripsy (IVL) technologies, has raised $36 million in a Series B financing round. The funds are expected to go toward supporting new clinical trials for the company’s Pulse IVL platform in addition to launching the platform’s eventual launch in the United States.
“We are pleased to see strong, sustained investor excitement around this technology,” Mark Toland, chairman of the board for AVS, said in a prepared statement. “IVL now represents a large and well-proven therapy that demands new solutions for patients with severely calcified arterial disease.”
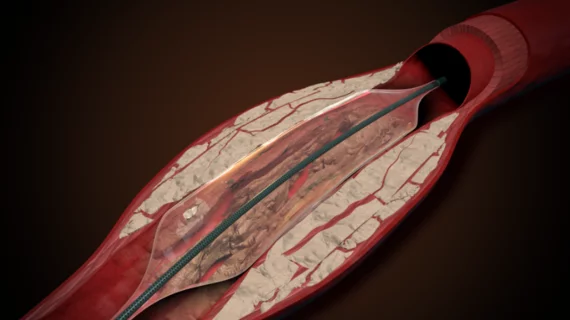
The Pulse IVL system works by delivering high-frequency pulsatile pressure waves at a rate of approximately 15 per second—and up to 5,000 pulses per case. Its design helps cardiologists break through the plaques without the use of an emitter. Instead, the pulses traverse the entire length of the balloon.
“Because the Pulse IVL device uses a unique mechanism of action that eliminates the need for electrical emitters, the deliverability, crossability and efficiency are optimized for very challenging and tortuous coronary cases,” Steven Yakubov, MD, medical director of the OhioHealth Research Foundation in Columbus and a member of the AVS Physician Steering Committee, explained in the same statement.
AVS is already enrolling patients into the POWER PAD II trial, which is tracking data from up to 120 U.S. patients with calcific femoropopliteal arteries who undergo treatment with the Pulse IVL platform. Two additional trials, one for coronary patients and another for carotid patients, are also in the works.
The Pulse IVL system has not yet been approved by the U.S. Food and Drug Administration (FDA).
IVL remains a top priority for medtech companies and care teams alike
IVL has quickly emerged as one of healthcare’s most in-demand technologies, allowing cardiologists to safely break up heavily calcified plaques and prepare patients for percutaneous coronary intervention.
After the runaway success of Shockwave Medical, now a part of Johnson & Johnson MedTech, several other companies are working to develop their own take on IVL. This latest funding round will certainly help AVS as it works toward securing FDA approval for the Pulse platform and officially entering this competitive market.