FDA announces another new recall involving Abbott’s HeartMate 3 LVADs after 70 injuries, 2 deaths
The U.S. Food and Drug Administration (FDA) has announced that Abbott is recalling nearly 900 left ventricular assist device (LVAD) implant kits due to ongoing safety concerns. This is a Class 1 recall, which means using these devices “may cause serious injuries or death.”
Abbott’s LVADs were involved in a separate Class I recall back in April.
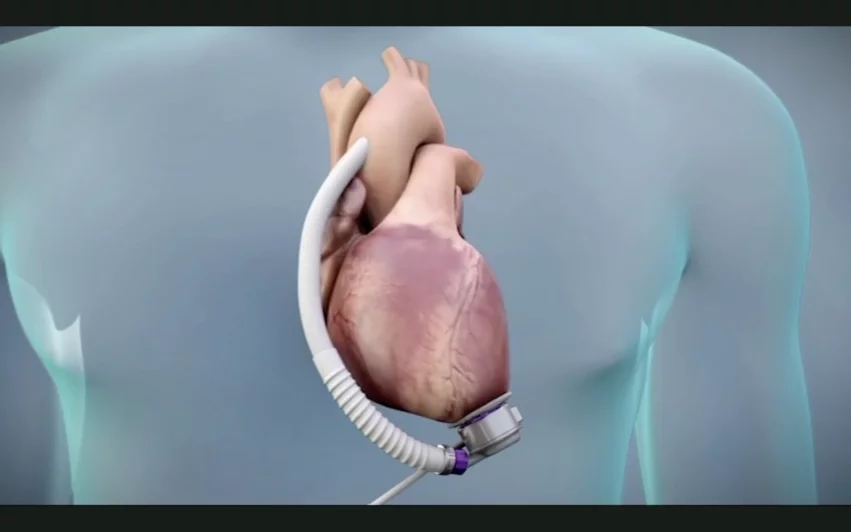
For this latest recall, Abbott is recalling nearly 900 HeartMate 3 LVAD implant kits due to repeated reports of blood leakage or air entering the device between the inflow cannula and apical cuff. This issue has only been seen during the initial implant of the LVAD.
“Blood leakage or air entering the LVAD from this location will impact the integrity of the blood flow and may lead to longer than expected surgery, bleeding (hemorrhage), right heart failure or air embolism,” according to the advisory. “Use of these devices may cause serious injury or death.”
Abbott has received 81 reports of this issue occurring during LVAD implantation. These incidents were associated with 70 injuries and two deaths.
The HeartMate 3 LVAD is approved by the FDA for the long- and short-term treatment of adult and pediatric patients with severe left ventricular heart failure. It is the only device of its kind currently available in the U.S. for this patient population. The HeartMate 3 LVAD works by pumping blood for the patient’s left ventricle, diverting it to other areas of the body. Cardiologists and cardiac surgeons often turn to these devices before or after a heart transplant. In some cases, it is viewed as a permanent destination therapy when nothing else will work. It is used both inside and outside of the hospital setting.
This recall is a correction, not a full product removal
The FDA is not asking customers to return these devices to the manufacturer. Instead, the agency is emphasizing the importance of following certain steps if blood leak or air entrainment is suspected during LVAD implants. Those steps include:
- Residual air must be completely evacuated from the device blood chamber prior to initiating the LVAD support.
- Ensure that bleeding is assessed and ensure proper management of hemostasis before closing all wounds.
- Use conventional strategies for resolving air leaks or surgical bleeding, including: adjusting the pump position, waiting for the natural tendency of blood to coagulate or upon reversal of anticoagulation, adding surgical materials, and exchanging the apical cuff, the pump, or both.
- Always have a complete backup system (implant kit and external components) available on-site and in close proximity during the implantation procedure for use in the event of an emergency.
Abbott first sent out an Urgent Medical Device Correction letter to customers about this issue on March 20. Click here for additional details from the FDA.
Abbott comments on latest Class I LVAD recall
“While rates of serious adverse events related to this issue are extremely low, Abbott is working with physicians to reiterate surgical instructions that can stop blood and air leakage prior to completing an LVAD implant,” Abbott told Cardiovascular Business in a statement. “We are also developing a change in the seal interface for the HeartMate 3 that will help address this issue.”
The company also emphasized that no products are being removed from the U.S. market and patients who have already received the device “are at no risk related to this issue.”