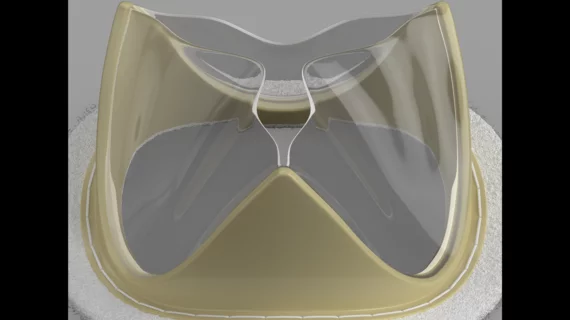
New-look polymer mitral valve linked to positive outcomes 30 days after surgery
Foldax, a Utah-based startup focused on developing surgical and transcatheter treatments for structural heart disease, has shared 30-day data on a new polymer mitral valve designed to limit calcification in younger patients presenting with severe valve disease.
The Tria surgical mitral valve is built using LifePolymer, a proprietary material that does not include animal tissue. Both the frame of the valve and its leaflets are generated by computer to match each patient’s native mitral valve. According to Foldax, this new polymer reduces the long-term risk of valve calcification, and patients should be able to bounce back after surgery without requiring the long-time use of anticoagulants.
Isaac George, MD, surgical director of the Heart Valve Center at Columbia University presented the 30-day data out of India at New York Valves: The Structural Heart Summit in New York City. The analysis included 67 patients between the ages of 19 and 67 years old. A majority of patients were women. After 30 days, the valves were associated with “excellent and stable hemodynamics,” according to Foldax. Additional research will examine patient outcomes after six months and then after one year.
“The LifePolymer material is uniquely formulated for heart valves and has been shown in animal studies to resist calcification, which can lead to stiffening leaflets that restrict blood flow through the valve,” George said in a statement. “Now, in the India study, imaging of the Tria mitral surgical valve in humans at 30 days shows no sign of calcifying or deteriorating leaflets. A durable non-animal tissue heart valve that may not require long-term anti-blood clotting medication could be transformative in providing new options for patients suffering from mitral valve disease.”
“We are encouraged by the strong clinical outcomes we are seeing with our patients in the Indian trial,” added principal investigator Kaushal Pandey, MD, a surgeon with P.D. Hinduja Hospital in Mumbai, India.
The Tria valve is not yet approved by the U.S. Food and Drug Administration.