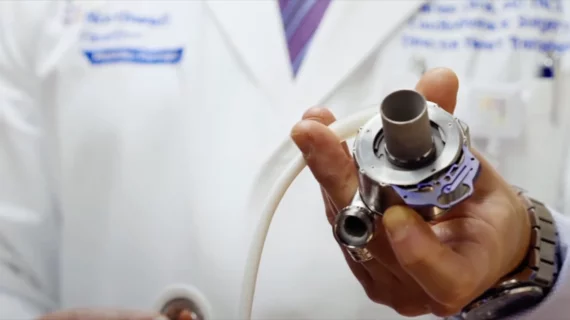
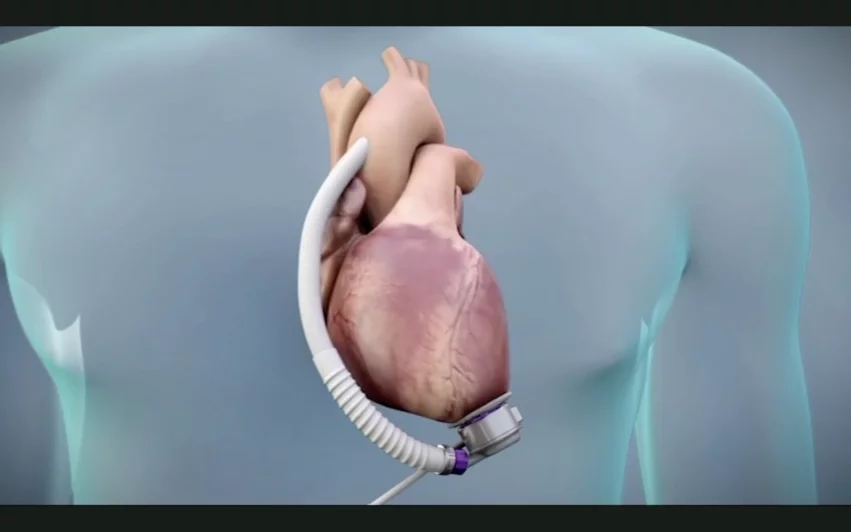
No aspirin, no problem: FDA approves key update for Abbott heart pumps
The U.S. Food and Drug Administration (FDA) has approved new labeling for Abbott’s HeartMate 3 left ventricular assist device (LVAD) that says aspirin is no longer recommended to help heart failure patients avoid post-implant blood clots. Prescribing an oral anticoagulant such as warfarin alone is enough to mitigate that risk, Abbott said, and the change could result in a significant improvement in patient outcomes. Regulatory agencies in Canada and Europe approved the same label update.
The shift was based largely on data from ARIES-HM3, an international study that found taking just warfarin and no aspirin after receiving a HeartMate 3 LVAD was associated with reduced risks of bleeding events and hospitalizations due to bleeding events.
“Aspirin, along with warfarin, has traditionally been mandated for advanced heart failure patients living with an LVAD, but whether it contributes to excessive bleeding has been uncertain,” Mandeep R. Mehra, MD, cardiologist, executive director of the Center for Advanced Heart Disease at Brigham and Women’s Hospital in Boston and principal investigator of ARIES-HM3, said in a statement. “The ARIES-HM3 trial, in which aspirin was removed from the medication regimen, provided important data challenging the assumption that patients with a heart pump must take aspirin daily. With this labeling change, physicians can avoid using aspirin in patients receiving the HeartMate 3 LVAD, a decision that is safe, and decreases bleeding and its associated hospital visits.”
“Removing aspirin from the medication regimen for the HeartMate 3 is a simple change that means people with an Abbott LVAD can focus on the things they love and spend less time worrying about and tending to bleeding events,” added Keith Boettiger, vice president of Abbott's heart failure business. “Through research such as the ARIES-HM3 trial, we continue to rewrite the book on the management of patients with advanced heart failure and focus on bringing life-enhancing benefits to people who rely on our devices to survive.”
HeartMate 3 at center of recent recalls
The Abbott devices have been at the heart of multiple FDA recalls in 2024. In April, for instance, customers were warned that the buildup of biological materials can cause issues. Instead of calling for the LVADs to be returned, the FDA urged customers to pay close attention to low-flow alarms.
The FDA announced a separate Class I recall in May after receiving multiple reports of blood or air entering the device during implantation. There was no call for the devices to be returned—instead, the FDA and Abbott emphasized the importance of following certain steps to avoid such issues.
Another Class I recall related to HeartMate LVADs was announced in June, related to the system monitors. Again, this was a product correction and not a full removal. Abbott shared recommendations for customers to help them avoid any issues going forward.