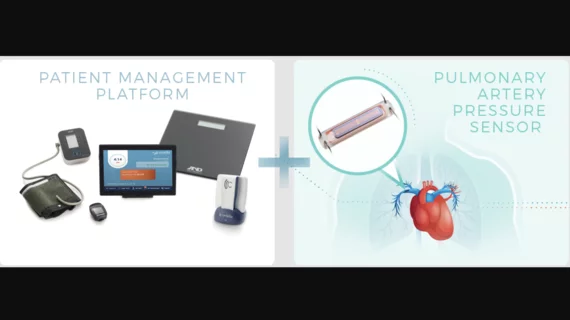
Implantable PA sensor for heart failure linked to positive 1-year outcomes
Endotronix, now an Edwards Lifesciences company, has shared updated data on the safety and effectiveness of its Cordella Pulmonary Artery (PA) Sensor System for managing heart failure patients.
According to new data from the PROACTIVE-HF study, treatment with the Cordella system was associated with significantly reduced risks of all-cause mortality and heart failure hospitalization after one year. The study, based on more than 450 patients presenting New York Heart Association (NYHA) class III symptoms, also tracked quality-of-life data, finding that Kansas City Cardiomyopathy Questionnaire scores were up 10.5% and six-minute walk scores were up 13.3% among Cordella patients.
“These data are consistent and compelling, validating that PA pressure-guided therapy improves heart failure outcomes,” PRACTIVE-HF principal investigator Liviu Klein, MD, MS, section chief of advanced heart failure, mechanical circulatory support, pulmonary hypertension, and heart transplant at the University of California San Francisco, said in a statement. “The trial results add to the growing understanding of the impact of comprehensive data—seated PA pressure and vital signs – to further improve outcomes, inform remote medical adjustments and directly engage heart failure patients in their own care. In the trial, clinicians reduced the PA pressures of congested patients by optimizing guideline-directed medical therapy and diuretics to improve heart function. And unique to Cordella, patients have visibility to their health data that helps drive their engagement and compliance.”
The Cordella system was approved by the U.S. Food and Drug Administration in June 2024, just weeks before Endotronix was acquired by Edwards. It uses an implantable sensor to tracks the patient’s PA pressure and other helpful health metrics, including blood pressure, pulse and various symptoms. PA pressure is known as an early indicator of congestion and can be used to determine when patients may need additional care.
Endotronix is already working on the PROACTIVE-HF 2 study. The company hopes to enroll up to 1,500 patients with NYHA Class II and III heart failure symptoms from the United States and Europe.