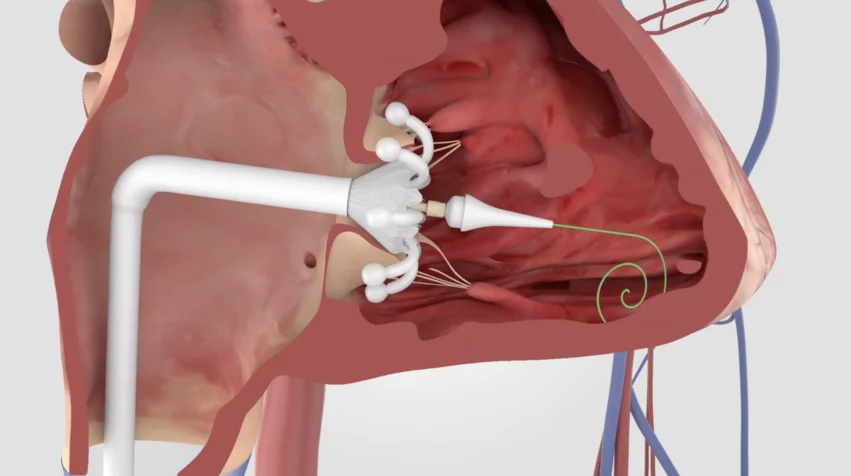
TTVR with Evoque device linked to substantial benefits after 1 year
Treating severe tricuspid regurgitation (TR) with the Evoque transcatheter tricuspid valve replacement (TTVR) system from Edwards Lifesciences is associated with significant improvements in symptoms and quality of life (QOL) after one year, according to new data presented at Transcatheter Cardiovascular Therapeutics (TCT) 2024 meeting in Washington, D.C.
Edwards made history in February when Evoque received U.S. Food and Drug Administration (FDA) approval for treating patients with symptomatic severe TR that does not improve after optimal medical therapy (OMT). It was the first device of its kind to gain FDA approval, finally giving U.S. patients with severe TR access to a a full transcatheter valve treatment option.
The FDA made its decision based on data from the TRISCEND II clinical trial, which found that treatment with Evoque was associated with significantly better outcomes than medical therapy alone. For this latest analysis, lead author Suzanne V. Arnold, MD, an interventional cardiologist with Saint Luke’s Mid America Heart Institute in Kansas City, Missouri, and colleagues investigated one-year QOL data from TRISCEND II patients.
Arnold shared her team’s findings during a late-breaking clinical trial session at TCT in front of a packed crowd. It was one of the most highly anticipated presentations of the entire conference.
Exploring one-year QOL data for the Evoque TTVR system
Arnold et al. reviewed data from nearly 400 patients with severe TR, including 259 who underwent TTVR with Evoque another 133 who were treated using OMT alone. The mean age for both groups was 79 years old, and a vast majority of patients in both groups were women.
Researchers tracked QOL by evaluating each patient’s Kansas City Cardiomyopathy Questionnaire Overall Summary Score (KCCQ-OS) over time.
Overall, QOL was consistently much better for patients who underwent TTVR after 30 days, six months and one year.
More than 56% of patients presented with massive or torrential TR. The KCCQ-QS improvements in those patients were twice as large for what the group saw in patients with severe TR, suggesting that they may benefit the most from transcatheter treatment.
These late-breaking findings were published in full in the Journal of the American College of Cardiology.[1] Click here to review the team’s analysis.
Evoque researchers excited to share data with cardiologists
“It is exciting to have the Evoque system available as a treatment option for patients who are very sick and otherwise have limited, if any, options,” TRISCEND II principal investigator, Susheel Kodali, MD, a professor with the Columbia University Vagelos College of Physicians and Surgeons and director of interventional echocardiography at the Structural Heart and Valve Center at New York-Presbyterian/Columbia University Irving Medical Center, said in a statement. “The one-year outcomes from the TRISCEND II trial demonstrate the benefits of this therapy in these patients and the favorable trends in all-cause mortality and heart failure hospitalization are encouraging to see. We are pleased to see TTVR reach this stage after nearly a decade of development.”
“Patients receiving TTVR with the Evoque system were twice as likely to be alive with a good QOL at one year, compared with the control group,” Arnold added.
Daveen Chopra, corporate vice president of transcatheter mitral and tricuspid therapies with Edwards Lifesciences, shared his own perspective on the late-breaking data.
“Edwards’ commitment to innovation is inspired by the millions of patients around the world suffering with debilitating symptoms and poor quality-of-life as a result of structural heart diseases and in desperate need of effective treatment options,” he said. “We are building a portfolio of transcatheter repair and replacement technologies for both the mitral and tricuspid valves, and we are dedicated to supporting those therapies with world-class evidence like what is being generated through the TRISCEND II trial.”