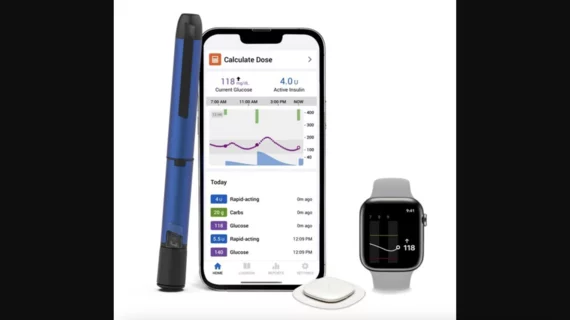
Medtronic receives key FDA clearance, launches new diabetes system
Medtronic has received U.S. Food and Drug Administration (FDA) clearance for its new InPen smartphone app and announced the launch of its new Smart MDI system. Smart MDI was designed to provide real-time insights to users on multiple daily injection (MDI) therapy. It includes the InPen smart insulin pen, the InPen app and Medtronic’s disposable, all-in-one Simplera continuous glucose monitor (CGM), which received U.S. Food and Drug Administration approval back in August.
The InPen app sends alerts to users who miss scheduled injections. MDI therapy can be challenging to track for some individuals—Medtronic built InPen, and the entire Smart MDI system, to remove some of those complexities.
“I'm thrilled about the launch of the Medtronic Smart MDI system with the InPen app and Simplera CGM,” Diana Isaacs, PharmD, BCPS, BCACP, BC-ADM, CDCES, a diabetes advocate and director of education and training in diabetes technology at Cleveland Clinic, said in a statement. “This is a significant leap forward for those on multiple daily injections, offering intelligent dosing insights and simplifying diabetes management. By reducing the guesswork out of insulin dosing, this tool helps maintain stable blood sugars, optimize long-term health, and reduce complications from hyperglycemia.”
Medtronic is starting out with a soft launch focused on existing CGM and InPen customers. That will then be followed by a more extensive commercial launch.
Medtronic and Abbott collaborating on new diabetes technology
In August, Medtronic announced a new partnership focused on improving care for patients with type 1 and type 2 diabetes. The two companies, typically seen as rivals in the competitive diabetes care landscape, are joining forces on a new CGM that will use Abbott’s own FreeStyle Libre sensors and work exclusively with Medtronic’s smart insulin delivery systems and software.
“Our partnership with Abbott allows us to expand access to our advanced automated insulin delivery and Smart MDI systems that deliver best-in-class outcomes with the most widely used CGM in the world,” Que Dallara, executive vice president and president of Medtronic’s diabetes division, said at the time.