Boston Scientific enters renal denervation market with acquisition worth up to $540M
Boston Scientific is officially jumping into the renal denervation (RDN) market. The company has agreed to acquire SoniVie, an Israeli medtech company developing its own RDN system to treat resistant hypertension and other hypertensive disorders. Boston Scientific is making an upfront payment of approximately $360 million, and additional payments of up to $180 million.
Boston Scientific was a strategic investor in SoniVie and already owned approximately 10% of the company. This transaction would cover the remaining 90%.
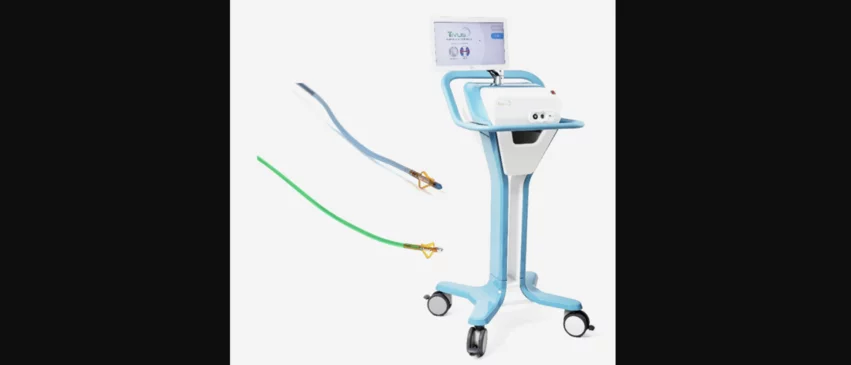
SoniVie’s Tivus intravascular ultrasound system works by targeting the nerves surrounding a patient’s blood vessels with “precise ultrasound energy”. The goal is to ablate the nerves in the renal arteries, which stops vasodilation and props the vessels in the fully open position to increase kidney filtration. The Tivus system has not yet been approved by the U.S. Food and Drug Administration (FDA) or any other regulatory authorities, but a global investigational device exemption (IDE) trial focused on its safety and effectiveness is now underway.
“RDN for hypertension is an exciting medical advancement for the millions of patients it may help and is supported by positive results from contemporary clinical trials and ongoing research," Lance Bates, senior vice president and president of interventional cardiology therapies with Boston Scientific, said in a statement. “We believe the addition of the differentiated, ultrasound-based Tivus system can complement our expansive interventional portfolio with a minimally invasive therapy for patients with hypertension and provides opportunity for future advancements in this space.”
This transaction is expected to close in the first half of 2025.
RDN systems gaining momentum
While RDN is not necessarily a cure for hypertension, it offers clinicians a new way to control its symptoms and reduce blood pressure in patients who do not respond to medication alone. RDN’s rise in recent years has been a major medtech story in recent years.
In November 2023, Recor Medical’s Paradise Ultrasound RDN system was the first RDN offering to gain FDA approval. Medtronic’s Symplicity Spyral RDN system was not far behind, gaining approval from the agency later that same month.
Both RDN systems went on to receive improved outpatient and inpatient from the U.S. Centers for Medicare and Medicaid Services in 2024, a sign that the technology is being embraced by healthcare provider and policymakers alike.
Another significant M&A deal for Boston Scientific
Boston Scientific has opened up 2025 with a series of big-dollar acquisitions designed to help the company make a splash in new medtech markets. In January, for example, the company agreed to acquire intravascular lithotripsy specialists Bolt Medical for up to $664 million. The deal includes an upfront payment of $443 million and up to $221 million in additional payments based on certain regulatory milestones.
Just like the SoniVie acquisition, Boston Scientific was already an early investor in Bolt Medical and owned some equity going into the transaction.
In 2024, meanwhile, Boston Scientific acquired stroke specialists Silk Road Medical for approximately $1.16 billion.