Cardiologists treat first commercial patients with FDA-cleared valve-in-valve TAVR device
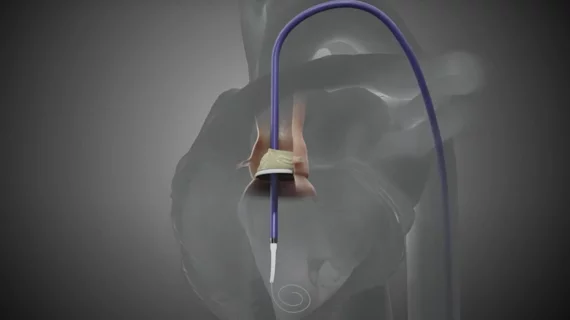
Pi-Cardia, an Israeli medtech company, announced that multiple heart teams throughout the United States have now used its ShortCut leaflet modification device to perform successful valve-in-valve transcatheter aortic valve replacement (TAVR) procedures.
These are the commercial patients to be treated with ShortCut, which gained U.S. Food and Drug Administration (FDA) clearance for valve-in-valve TAVR in September, after receiving the agency’s breakthrough device designation in January 2024.
The procedures were performed by Philippe Généreux, MD, and Gennaro Giustino, MD, with the Gagnon Cardiovascular Institute; Raj Makkar, MD, and Wen Cheng, MD, with Cedars-Sinai Medical Center; and Vinod H. Thourani, MD, and Pradeep Yadav, MD, with the Piedmont Heart Institute.
“We are honored to have performed the first commercial ShortCut procedure and to be at the forefront of the innovative field of leaflet modification,” Généreux said in a statement. “In the past, treating this challenging patient population was possible mainly with surgical approaches or complex techniques. With ShortCut, we safely split both the right coronary cusp and left coronary cusp leaflets in a simple, predictable and controlled manner, enabling safe placement of a TAVR.”
“The ShortCut device is a true game changer, addressing one of the most significant challenges in structural heart,” added Makkar. “It will play a fundamental role in the lifetime management of aortic stenosis patients who may require multiple valve interventions. Given its quick and intuitive nature and seamless integration into the standard TAVR workflow, I am confident this tool will become highly adoptable across TAVR centers.”
ShortCut, described by Pi-Cardia as the “world’s first dedicated leaflet modification device,” is used to split valve leaflets when patients present with an increased risk of coronary obstruction. The company is also developing a similar device, ShortCut Mitral, for treating high-risk transcatheter mitral valve replacement patients.
“We are incredibly proud of this significant milestone and are excited to pioneer the leaflet modification market,” Erez Golan, Pi-Cardia’s CEO, said in the same statement.