FDA approves design updates for Perceval Plus surgical aortic valve
Corcym has received U.S. Food and Drug Administration (FDA) approval for a new version of its Perceval Plus surgical aortic heart valve that includes the company’s advanced LANCELOT features.
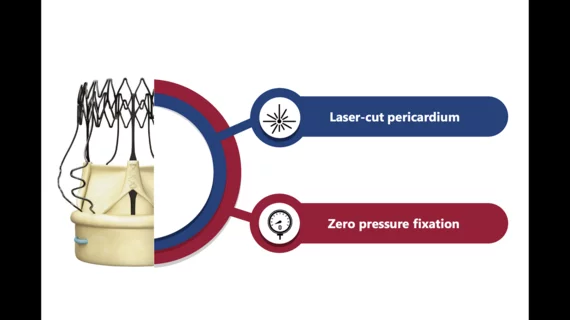
The Perceval Plus with LANCELOT surgical heart valve, like the Perceval Plus before it, is designed to be implanted during surgical aortic valve replacement (SAVR) procedures. Its sutureless, collapsible frame was built to simplify implantation and help patients recover faster following surgery. This updated version includes two key new details: laser-cut leaflets and thread holes, designed to improve consistency, and a zero-pressure fixation process that reduces stress on the leaflets.
“For many years, Perceval has been well-known as the only sutureless valve designed to simplify surgical implantation, reducing the impact of surgery with faster patient recovery,” Christian Mazzi, Corcym CEO, said in a statement. “We expect LANCELOT technologies to take it a step further, delivering the next generation of reproducibility and consistency in valve performance for cardiac surgeons and their patients around the world.”
While many medtech companies have started focusing more and more on transcatheter aortic valve replacement, Corcym remains dedicated to developing and distributing SAVR valves for the cardiac surgery market.
“We at Corcym are committed to helping cardiac surgeons excel and treat their patients better today and in the future, worldwide,” Mazzi added. “We are pleased to bring these new surgical aortic valve technologies as a treatment option to patients in the U.S.”