FDA grants breakthrough device designation to AI-powered, retinal imaging-based CVD assessments
Toku, a New Zealand-based medical device company focused on retinal imaging technology, has received a breakthrough device designation from the U.S. Food and Drug Administration (FDA) for its CLAiR platform.
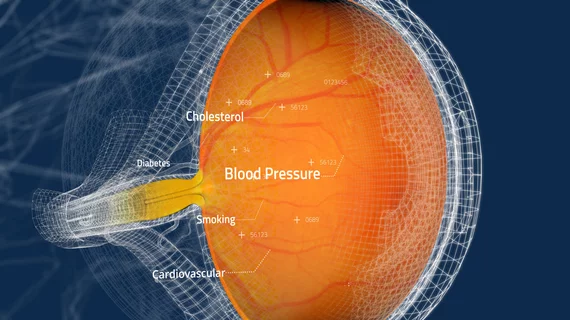
CLAiR offers physicians a new away to evaluate a patient’s cardiovascular health using non-invasive retinal images captured during routine eye exams. Because the retina represents the only transparent part of the human vascular system, Toku began focusing on ways to photograph it quickly and easily.
CLAiR uses advanced artificial intelligence (AI) algorithms to provide real-time cardiovascular disease assessments, examining retinal images for signs of high cholesterol, hypertension or other risk factors. The technology was designed to be integrated with the retinal imaging cameras eye doctors already have equipped in their offices.
“Toku’s mission is to make identifying disease accessible for everyone, everywhere, all the time,” Ehsan Vaghefi, CEO and co-founder of Toku, said in a statement. “The breakthrough device designation that the FDA has granted to our CLAiR technology platform is a validation of the tremendous potential our CLAiR AI technology can provide to the tens of millions of patients who may unknowingly be at risk of a devastating cardiovascular condition. This designation greatly de-risks our clinical development and regulatory pathway for the technology, as the FDA’s breakthrough devices program offers medical device companies accelerated review processes, enhanced guidance, and prioritized evaluation, facilitating quicker market access for innovative technologies and encouraging the development of devices that significantly improve patient care.”
“Predictive analytics technology, provided it has been proven to be accurate and applicable to the population using it, has tremendous potential to benefit patients by identifying those at highest risk so treatments and preventive measures can be initiated quickly,” added April Maa, MD, a professor of ophthalmology with Emory University School of Medicine. “The medical literature has published many examples using different types of eye imaging to predict risk of other systemic conditions beyond cardiovascular disease, such as neurologic or kidney disease. Therefore, further developing this type of technology is very exciting as cardiovascular disease remains a significant cause of morbidity and mortality in the whole country,”