ASE updates fetal echocardiography guidelines
The American Society of Echocardiography (ASE) has issued an updated 44-page guideline for fetal cardiac ultrasound.
Fetal echocardiography is a highly sensitive and specific noninvasive tool used to detect, classify and evaluate fetal cardiovascular diseases. The new document, "Guidelines and Recommendations for Performance of the Fetal Echocardiogram: An Update from the American Society of Echocardiography," replaces an older ASE guideline on the topic from 2004.
“Ongoing research and collaboration have supported efforts toward a better understanding of fetal physiology and disease processes and progression. This has led to significant improvements in fetal diagnosis and in clinical practice and outcomes for patients,” co-chair of the writing committee Anita J. Moon-Grady, MD, director of the University of California San Francisco fetal cardiovascular program, said in a statement. “The information included in the updated guideline will help practitioners and clinicians maintain the best practice for fetal echocardiography and fetal and perinatal cardiovascular care across disciplines.”
ASE explained in its statement that the standards for imaging, reporting and communication of test results in fetal echocardiography continue to advance rapidly, and a considerable amount of new information and new technology has become available in the last 20 years. The society said the purpose of this new guideline document is to provide updated recommendations for the performance and interpretation of fetal echocardiography.
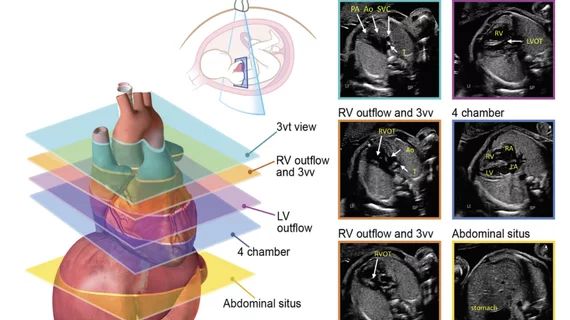
The guideline focuses on the detection, classification, risk assessment, and perinatal care planning of pregnancies where the fetus has cardiovascular disease.
It covers indications for imaging, how to perform the exams, cost-effectiveness for screenings, a list of required measurements and illustrations showing what echo biometrics should be quantified, what to look for during various gestational periods and guidance for disease-specific anatomic, physiologic and functional evaluation for commonly encountered fetal congenital heart disease (CHD) lesions.
The guideline’s writing group was commissioned by ASE’s Pediatric and Congenital Heart Disease Council and lead by co-chairs Moon-Grady and Mary T. Donofrio, MD. The group presents detailed guidelines for what constitutes a complete fetal echocardiographic examination that can be used as a guide for both learners and experienced practitioners. The Fetal Heart Society, the Society of Pediatric Echocardiography and the American Institute of Ultrasound in Medicine also endorsed the document, which is published in the July 2023 issue of the Journal of the American Society of Echocardiography.[1]
This document and all guidelines published by ASE are available at ASEcho.org/Guidelines.