FDA clears smaller 3D TEE transducer for imaging children, high-risk adult patients
Philips Healthcare received U.S. Food and Drug Administration (FDA) clearance for its compact X11-4t Mini 3D transesophageal echocardiography (TEE) ultrasound transducer.
The transducer was designed to improve image quality when evaluating certain patient populations, including pediatric patients and adults who present with a heightened risk of complications. Acquiring 3D TEE images is often a crucial step when evaluating these patients, but performing those tests can prove challenging in some patients using current, larger sized TEE probes.
The new device is 35% smaller than previous Philips offerings, and its “pill-shaped” design is seen as a key improvement in terms of patient comfort. In addition, Philips says any operators with prior experience using the company’s echocardiography solutions should require “minimal additional training” to feel at ease with this new technology.
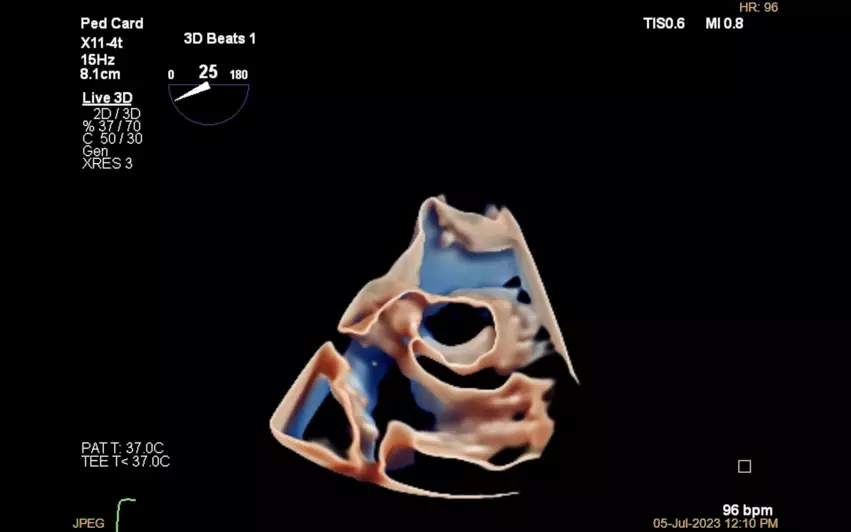
“In many of our smallest patients undergoing complex intracardiac procedures like valve repairs, 3D TEE will give us a new and much needed perioperative tool,” Brian Soriano, MD, a pediatric cardiologist with UW Medicine in Seattle, said in a prepared statement. “For example, the X11-4t can help us visualize atrioventricular valves en-face. In many cases, this is a view that is difficult to achieve with traditional 2D TEE. 3D TEE will also be a more effective tool to communicate with the surgeons and will enable us to give good ‘surgeon views’ of intracardiac structures.”
“With its excellent image quality and small footprint, the X11-4t transducer has the potential to reduce the complications of prolonged transesophageal imaging which can occur during our most difficult structural heart procedures,” added Rebecca Hahn, MD, a professor with Columbia University Irving Medical Center and director of interventional echocardiography with the Columbia Structural Heart and Valve Center. “The transducer’s small size may also be better tolerated by patients during shorter procedures performed under conscious sedation and thus, provide additional high-quality imaging to improve procedural outcomes without the need for general anesthesia.”
According to Philips, the X11-4t Mini 3D TEE transducer is compatible with its full ultrasound portfolio, including the Epiq CVx and EchoNavigator.
The company expects this technology to be commercially available in the United States in the near future. It is presently working toward receiving CE mark approval in Europe.
Hahn is known as a leading expert in echocardiography. Cardiovascular Business interviewed her at ASE 2023 about the evolving role of interventional echocardiographers.