Cardiologist sees ‘tremendous potential’ in new-look PFA system
A new pulsed field ablation (PFA) system from Kardium could give cardiologists a new tool in the war against atrial fibrillation (AFib), according to new data published in Heart Rhythm.[1]
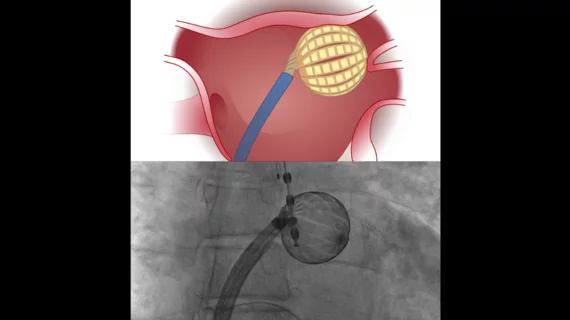
The first-in-human PULSE-EU trial focused on 48 patients who presented with paroxysmal (48%) or persistent (52%) AFib. All patients were treated at a single hospital from September 2022 to January 2023 with the Globe Pulsed Field System, a spherical array, single-shot PFA catheter that delivers biphasic pulses. Ablation targets included the pulmonary veins, posterior walls and mitral isthmuses.
Overall, the success rates were 100% for pulmonary vein isolation, 100% for the posterior walls and 91% for the mitral isthmuses. Mean pulse delivery time, left atrial catheter dwell time, fluoroscopy time and procedure time were 61.5 seconds, 53.9 minutes, 5.3 minutes and 87.8 minutes, respectively.
Freedom from atrial arrhythmia after one year was 84.2% for paroxysmal AFib and 80% for persistent AFib.
“I am very excited by the results of the PULSE-EU Study with the Globe Pulsed Field System,” Vivek Reddy, MD, director of cardiac arrhythmia service with The Mount Sinai Hospital in New York City, said in a statement. “The Globe System achieved outstanding freedom from atrial arrhythmia in both Paroxysmal and Persistent patients, with no device- or procedure-related major adverse events. These outcomes underscore the tremendous potential of the Globe System in advancing AFib treatment and improving patient outcomes.”
“These findings highlight the potential of the Globe System to set new standards in the treatment of AFib, with the potential to offer a safe and more effective therapeutic option for patients,” added Kevin Chaplin, Kardium’s CEO. “Everyone at Kardium looks forward to further advancing our Globe technology and making it available for patients worldwide.”
Reddy presented the PULSE-EU trial data to attendees at Heart Rhythm 2024 in Boston, the annual meeting of the Heart Rhythm Society. Click here for additional Cardiovascular Business coverage from the conference, including exclusive video interviews.
Kardium is a Canadian medical device company focused on electrophysiology technologies. The Globe Pulse Field System is not yet approved by the U.S. Food and Drug Administration.
Click here to read the full analysis.