Medical device startup gains FDA clearance for new RF guidewire, raises $12.5M
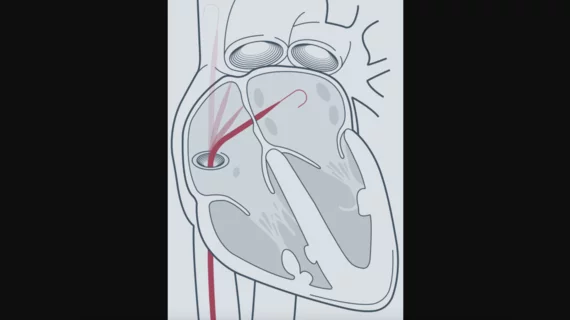
Atraverse Medical, a San Diego-based medical device company focused on left-heart technology, has received U.S. Food and Drug Administration (FDA) clearance for its Hotwire radiofrequency (RF) guidewire.
The Hotwire RF guidewire is designed to provide precise left-heart access to electrophysiologists while serving as a rail for catheter-based therapy. According to Atraverse, it offers “universal sheath compatibility,” allowing users to stick with the technology they prefer during RF procedures.
“The FDA clearance of the Hotwire underscores our dedication to medical innovation and our commitment to improving the standard of care for procedures requiring transseptal access including endocardial ablation, left atrial appendage closure, and mitral valve repair,” electrophysiologist Steven Mickelsen, MD, Atraverse co-founder and chief translational science officer, said in a statement.
“I am excited about the Hotwire’s ability to drive impactful change in the field of left-heart therapies, allowing physicians to use their preferred transseptal access workflow with the potential for safer, faster procedures,” added Devi Nair, MD, director of cardiac electrophysiology and research at St. Bernard’s Medical Center and an early user of the newly approved device.
Atraverse Medical raises $12.5 million
Receiving clearance from the FDA was not the only good news Atraverse had to share, the company also raised $12.5 million in an initial seed round that brought in more funding than it originally expected. The fresh investments are expected to help fund research and development in addition to the early commercialization of the Hotwire guidewire.
The device specialists behind Atraverse are no strangers to this type of success. The company was founded in 2022 by the group that founded Farapulse, which was eventually acquired by Boston Scientific for an upfront payment of $295 million. The Farapulse technology is now one of Boston Scientific’s trademark offerings after receiving FDA approval to treat patients with symptomatic paroxysmal atrial fibrillation in January.