A glimpse into the future: FDA has cleared multiple AR, VR tools for cardiology
Examples of virtual reality (VR) and augmented reality (AV) have been seen at many cardiology conferences in recent years. These technologies may seem like something from the future, but many are already cleared by the U.S. Food and Drug Administration (FDA). The FDA released a list of 69 clinical VR and AR products it has granted clearance to since 2015, and that number is expected to grow as time goes on. A third of the FDA approvals were granted in just the last 18 months.
The list includes two products specific to the cardiology, but several others listed under radiology are also aimed at cardiology. This includes technologies offering true 3D image assessments, procedural planning and guidance. The basic concept of all of these software systems is to convert segmented medical imaging from a patient into true 3D objects to enable physicians to better understand complex anatomy, plan, practice and guide procedures. Some of the offerings can also help with patient education.
Augmented reality helps guide EP lab procedures
SentiAR Inc. has the two applications specific to the cardiac EP lab. The SentEP system, cleared in September 2020, can project multi-dimensional digital images in an augmented reality headset that still allows an electrophysiologist to see their surroundings, tools and the patient. The 3D projections are meant to help with intraprocedural device guidance.
The second approval for SentiAR was in June 2023 for the CommandEP Data Manager PC, which enables the review, analysis and communication of multi-dimensional digital images for intraprocedural use. The approval also covered CommandEP, which allows the EP to visualize electro-anatomic data from the electro-mapping system during ablations.
This technology has been discussed in sessions and demonstrated on the expo floor at the Heart Rhythm Society (HRS) for the last couple years.
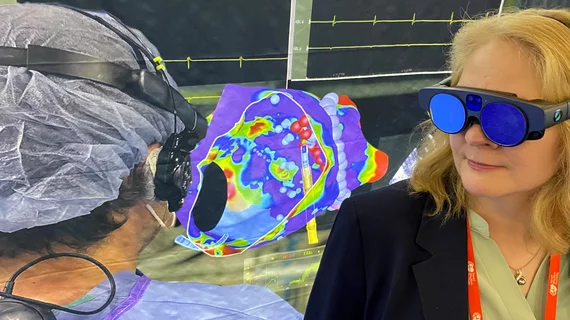
Augmented reality was recently integrated with GE Healthcare's Allia IGS angiography imaging platform through a partnership with MediView XR Inc. It enables interventional cardiologists to look at true 3D images and manipulate them with hand movements without breaking the sterile field. Photo courtesy of GE Healthcare.
Interventional cath lab AR procedural guidance
Similar augmented reality systems are being developed to help guide procedures in cath labs for interventional cardiology and interventional radiology.
GE Healthcare announced in June 2024 it partnered with MediView XR Inc. to integrate its OmnifyXR Interventional Suite interventional augmented-reality solution into GE's angiography systems and cath lab installs. The technology, cleared by the FDA in July 2023, fits seamlessly into existing interventional suites and leverages augmented reality to support workflow efficiency and ergonomics, improved visualization and collaborative care.
The first clinical use of this GE/MediView integration took place for two interventional radiology procedures earlier this year.
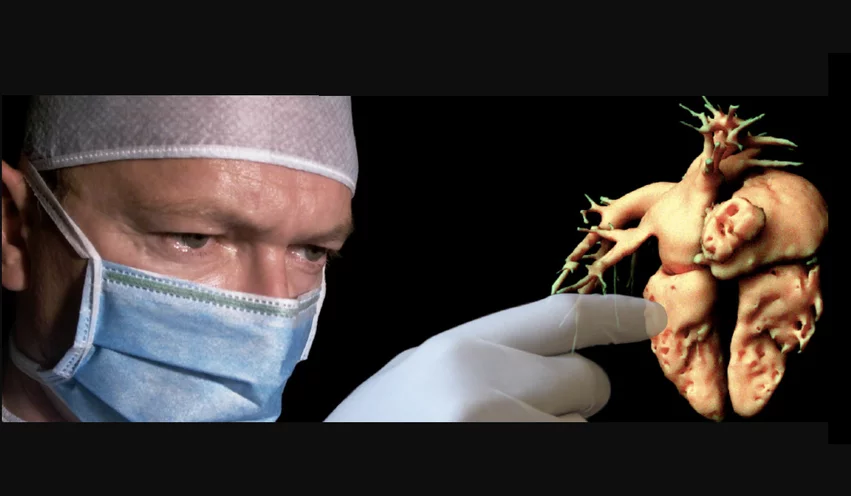
EchoPixel Inc. developed a AR system to view true 3D radiology imaging and 3D echocardiography images using special glasses. It was actually the first clinical AR technology to be cleared by the FDA in 2015. The company had a goal to use new visualization technology to eliminate the need for glasses or a headset and view holograms of real-time transesophageal echo (TEE) for us in structural heart procedures.
The vendor demonstrated this technology for physicians last year during the 2023 Transcatheter Cardiovascular Therapeutics (TCT) conference. A handful of U.S. physicians have also used it to guide transcatheter left-atrial appendage (LAA) closure procedures. The company has targeted the technology for structural heart, congenital heart and cardiac surgery.
Israeli start-up Real View Imaging Ltd. has also developed a technology to project holograms in mid-air that eliminate the need for wearing an AR headset or 3D glasses. The system allows interventional cardiologists to use their hands and fingers to manipulate and rotate the images without breaking the sterile field.
The company gained FDA 510(k) clearance for its Holoscope-i holographic system in May 2021. The system creates spatially accurate 3D interactive medical holograms from CT, rotational angiography acquisitions and 3D ultrasound.
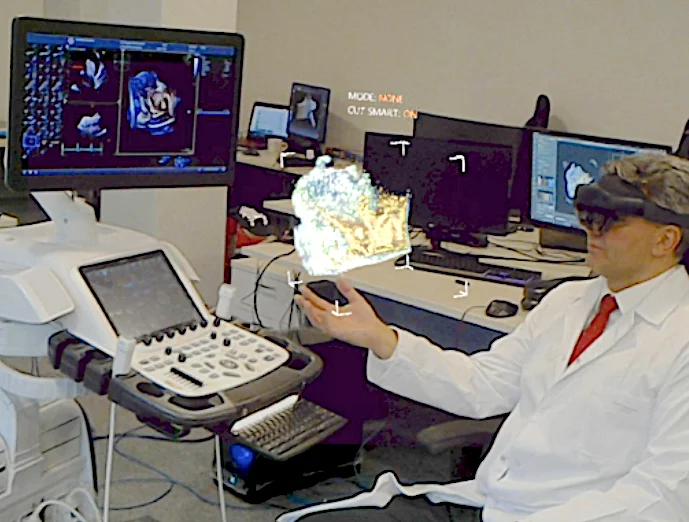
Philips Healthcare has been developing an AR system for the cath lab that uses a Microsoft HoloLens headset for interventional radiology and interventional cardiology. It was shown as a work-in-progress in a cath lab room display at the 2019 Radiological Society of North America (RSNA), but it is not yet commercialized.
Real-time holographic echo imaging
In addition to EchoPixel, Polish vendor MedApp has developed its CarnaLife Holo AR technology to view medical imaging and live 3D echo imaging. The FDA cleared the technology in May 2023. The vendor said it is working with GE Healthcare to integrate the technology into the Vivid E95 ultrasound system, where it will provide real-time 3D visualization from the ultrasound transducer and display it as interactive holograms.
Virtual reality may change cardiac surgery consults
Avatar Medical's Avatar Medical Software V1, cleared by the FDA in May 2023, can take cardiac imaging and create virtual reality files, potentially changing what consultations look like for patients and surgeons alike. Another benefit of using this technology could be enhancing learning experiences for medical students.
Sira Medical, meanwhile, gained FDA clearance in February 2024 for its AR preoperative surgical planning application that creates patient-specific, high-fidelity 3D holograms. The technology can also be used to help patients understand the anatomy and procedure better. It is aimed at several surgical use cases, including cardiology.
Several radiology AR applications are geared toward neuro and complex thoracic or abdominal surgical planning and guidance applications. A couple also center on needle tracking for interventional radiology procedures. Both of these technologies could have applications for cardiovascular surgery, guiding pericardiocentesis or vascular access.
Search the entire FDA list if AR and VR cleared clinical products.