FDA approves GE HealthCare's flurpiridaz F-18 PET radiotracer for CAD
The U.S. Food and Drug Administration (FDA) has granted a long-awaited approval to flurpiridaz F-18, GE HealthCare's first-of-its-kind positron emission tomography (PET) myocardial perfusion imaging radiotracer, for the detection of coronary artery disease (CAD).
GE HealthCare said it will market the new radiotracer under the trade name of Flyrcado.
Flurpiridaz is seen as a major step in advancing nuclear imaging technology. It was one of the hottest topics at the American Society of Nuclear Cardiology (ASNC) annual meetings in 2022, 2023 and 2024. Key cardiac imaging thought leaders interviewed by Cardiovascular Business in recent years have pointed to flurpiridaz as something important to keep an eye on.
Flurpiridaz is indicated for patients with known or suspected CAD. It also delivers higher diagnostic efficacy compared to single-photon emission computed tomography (SPECT) MPI, the primary exam currently used in nuclear cardiology. Flurpiridaz, which can be manufactured in an offsite pharmacy and delivered as a ready-to-use unit dose, has the potential to expand clinician and patient access to PET MPI, including improving diagnostic accuracy in difficult-to-image patients, such as those with a high body mass index (BMI) and women, GE HealthCare said.
It has a half-life of 109 minutes, significantly longer than existing PET MPI tracers. The most widely used PET tracer is rubidium-82, which requires an onsite generator, because the half-life of the tracer is only 75 seconds. Rubidium-82 also requires the use of pharmacologic stress rather than treadmill stress, which is preferred, so flurpiridaz brings the first practical opportunity to combine exercise stress testing with cardiac PET imaging for CAD, enabling the most robust protocol for evaluating ischemia in patients. Clinicians also have the ability to rescan a patient during the same imaging session in the event of technical difficulties, rather than rescheduling an additional scan.
“Flurpiridaz will be a game changer in the noninvasive diagnosis of CAD and assessment of myocardial blood flow. And the whole field of nuclear cardiology and cardiology have been waiting for a long time to get to this point. A large group of patients don't have access to this type of PET imaging for myocardial perfusion imaging. Flurpiridaz will make it possible so a larger group of patients will have access to this new technology,” explained Jamshid Maddahi, MD, principal investigator of clinical trials focused on flurpiridaz and a professor of cardiology and molecular and medical pharmacology at UCLA School of Medicine, said in an interview with Cardiovascular Business.
PET adoption widely expected to expand with flurpiridaz
While image quality is better with PET and SPECT, the costs and access to radiotracers are key barriers to access.
"Although PET MPI as a modality enables high diagnostic accuracy as compared to SPECT MPI, only a minority of annual PET scans in the U.S. are PET MPI because of limited access to the currently available PET tracers—which may be addressed with the introduction of Flyrcado," Maddahi said
Numerous, luminary cardiac imagers have predicted flurpiridaz will pave the way for an expansion of PET adoption. The FDA clearance of flurpiridaz is considered a paradigm shift for PET nuclear imaging because it offers improved imaging and delivers unit doses on demand that are more economical than the use of rubidium-82 generators, which can cost around $40,000 per month. This has discouraged lower volume centers from adopting PET. Now radiotracers can be ordered as needed for regional pharmacies.
“I believe that there will be increasing use of PET, but as we have seen in the past, when a new technology becomes available, it takes time to transition into the routine practice. I would anticipate that during the next five years, SPECT will still be done. I would anticipate that PET with rubidium will continue to be done. But, the numbers will diminish in favor of flurpiridaz. In my opinion, flurpiridaz is not here to replace just the existing technology, but it is opening new frontiers for access of patients that they did not have access to imaging with PET in the past,” Maddahi explained.
Mouaz Al-Mallah, MD, MSc, immediate ASNC past president and director of cardiac PET at Houston Methodist Hospital, has been a big advocate for upgrading nuclear cardiology programs to PET and said in previous interviews with Cardiovascular Business that flurpiridaz could help tip the balance in favor of PET investments.
“Given the desirable properties of flurpiridaz F-18, both in terms of myocardial uptake and fluorine-18 imaging characteristics, Flyrcado represents a favorable combination of imaging agent pharmacology and convenience to imaging institutions and patients. There are new frontiers for cardiac PET that this tracer can achieve. It can be ordered as a unit dose, and it offers the flexibility to perform exercise stress testing. We expect new imaging centers to be able to offer cardiac PET to their patients, making it more convenient to access PET MPI and providing a meaningful impact for clinicians and their patients," Al-Mallah said in a statement.
F-18 readily attaches to mitochondria in cells, and the heart has a dense concentration of mitochondria because of the amount of energy needed to keep the heart pumping. GE HealthCare said this makes F-18 agents very well suited for myocardial imaging.
ASNC and GE HealthCare both said proposed changes to the 2025 Medicare Physician Fee Schedule (MPFS) and the Hospital Outpatient Prospective Payment System (HOPPS) proposed rules that the Centers for Medicare and Medicaid Services (CMS) published last week. CMS is proposing to change the way it pays for diagnostic radiopharmaceuticals. Currently, the costs associated with diagnostic radiopharmaceuticals are packaged into payments for nuclear imaging tests. CMS now proposes reforming the current bundling policy to pay separately for any diagnostic radiopharmaceutical with a per-day cost greater than $630. This could increase the revenue from PET and prevent losses that would otherwise deter hospitals from adopting new PET programs.
Pivotal AURORA trial showed big advantage over SPECT
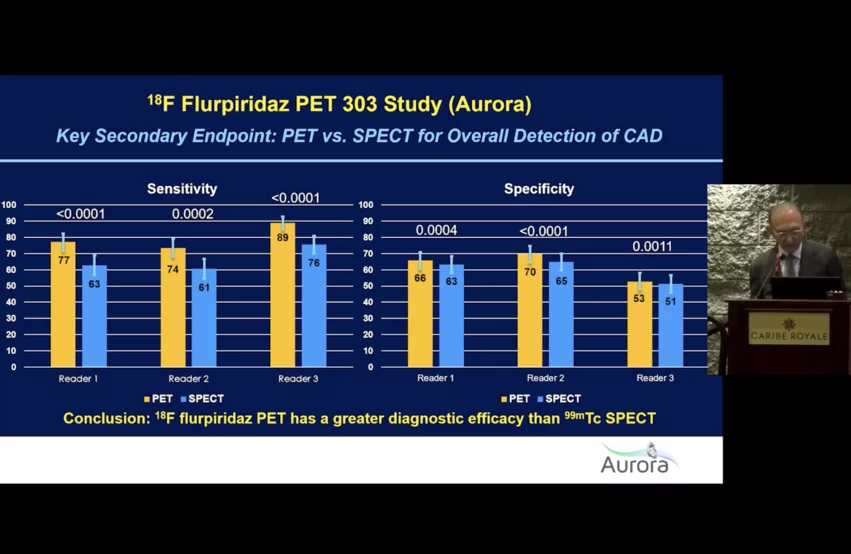
During the multicenter international AURORA Phase III trial, flurpiridaz F-18 was compared with both coronary angiography as a standard of truth to determine diagnostic efficacy in detecting CAD in addition to SPECT MPI.
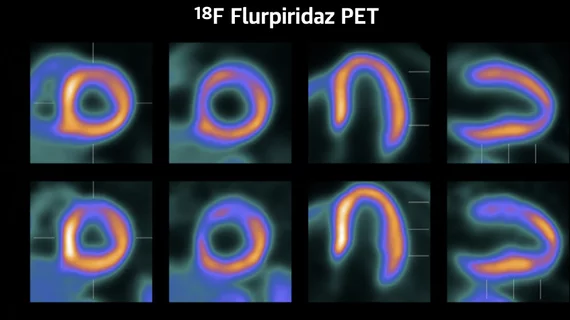
The trial found flurpiridaz PET was clearly superior in all areas assessed compared with more commonly used SPECT nuclear imaging. The results of the phase 3, open-label, multicenter study of flurpiridaz found much greater sensitivity and specificity than SPECT in all areas and types of patients compared.
"Flurpiridaz has a greater diagnostic efficacy that technetium labeled SPECT," Maddahi explained. "On average, there were 75% larger defects on PET compared to SPECT."
Maddahi said in some cases, this improved diagnostic sensitivity changes the course of patient care, when SPECT imaging of the same patients showed the perfusion was normal and the flurpiridaz PET showed clear perfusion defects.
In the 298 patients with a BMI equal to or greater than 30, he said flurpiridaz also had a greater diagnostic efficacy than SPECT.
Find more details from the Aurora study.
A big addition for GE HealthCare’s PET portfolio
Flyrcado is one of three F-18 imaging agents in GE HealthCare’s portfolio of FDA-approved molecular imaging PET products. It will join radiopharmaceutical PET imaging agent fluoroestradiol F-18 (Cerianna) injections, used to detect estrogen receptor positive lesions as an adjunct to biopsy in patients with metastatic and recurrent breast cancer. The other agent is flutemetamol F-18 (Vizamyl) injections, a PET tracer for imaging of the brain to estimate beta amyloid neuritic plaque density in adult patients who are being evaluated for Alzheimer’s disease or other causes of cognitive decline.
GE HealthCare acquired exclusive global commercialization rights for flurpiridaz F-18 from Lantheus in 2017 and has led its funding and development. Lantheus collaborated on the development and will also collaborate on commercialization through a joint steering committee. Lantheus is entitled to royalties based on commercial sales milestones.
Flyrcado will be available in initial U.S. markets in early 2025 with expanding availability thereafter. GE HealthCare said it is working with numerous contract cyclotron vendors that already produce its other F-18 agents so that flurpiridaz F-18 will be available to 90% of regions where there are centers currently offering PET by the end of 2025.