High dosage of blood thinners lowers 30-day morbidity for hospitalized COVID-19 patients
COVID-19 patients who were hospitalized, but not yet requiring intensive care, showed a 30% reduction in the risk of death within 30 days when given a treatment-strength dose of blood thinners compared with patients who received a lower, preventative-strength dose, according to a recent study published in the Journal of the American College of Cardiology and presented as a late-breakling trial at the American College of Cardiogy (ACC) 2023 annual meeting.[1]
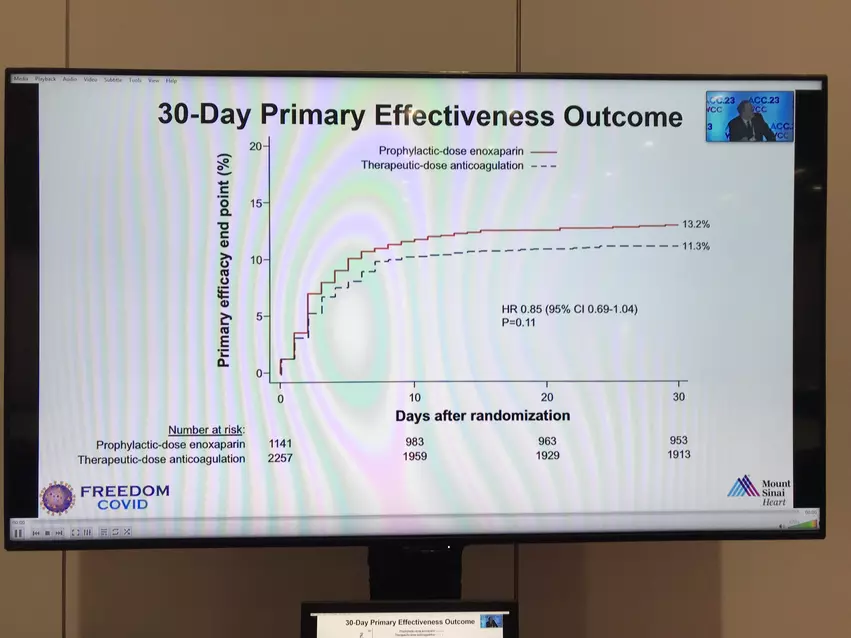
The study, known as the FREEDOM-COVID trial, initially set out to explore the impact of high-dose blood thinners on overall combined outcomes, to include any of the following: risk of death from any cause, need for hospitalization in an intensive care unit (ICU), blood clot, or stroke within 30 days. However, after finding that there was no significant difference in combined outcomes for patients who received the higher treatment-strength dose versus the lower preventative-strength dose, the researchers went on to explore the impact on each of the individual outcomes, discovering the lower 30-day morbidity rates.
What they found was a 4.9% 30-day morbidity rate for patients receiving the higher dose, compared with 7% for those receiving the lower dose. Additionally, the patients on the higher dose were significantly less likely to need a breathing tube than those on the lower dose, with respective rates of 6.4% versus 8.4%.
"Although our study did not reach its primary endpoint, it has shown that patients admitted to the hospital for COVID-19 who are not yet critically ill, but who have early signs of lung damage caused by the virus, substantially benefit from a higher dose of blood-thinning medication to stop disease progression, prevent the need for lung intubation, and prevent death, without a significant increase in the risk of bleeding," said Valentin Fuster, MD, PhD, president of Mount Sinai Heart, physician-in-chief of Mount Sinai Hospital, and the study's principal investigator.
Some of the high-dose patients received the blood thinners in the form of enoxaparin by injection, while others took them orally as apixaban. Both forms proved to be equally effective, which the authors note can be helpful due to the greater ease of use for orally-administered blood thinners. All of the low-dose patients received enoxaparin by injection.
Overall, the authors note, the anticoagulants appeared to have the greatest mortality-related for patients who showed early signs of COVID-related lung damage via X-ray or CT scan.
"For patients already critically ill with COVID-19 and on a breathing tube, the use of a treatment-strength blood thinner is unlikely to be beneficial, but for patients with less advanced lung damage or other high-risk features, this treatment may be lifesaving,” said Gregg W. Stone, MD, professor of medicine at the Icahn School of Medicine at Mount Sinai and co-author of the study.
The numbers are based on the outcomes for 3,398 patients across 10 countries who were randomly assigned to the different dosages.
View the full study here.