Cardiologists detail world’s first implant of new device for ‘no-option’ chest pain patients
Interventional cardiologists in Canada have performed the world’s first implant of a new coronary sinus reducer designed to treat chest pain patients who see no benefits from other available interventional or surgical treatments.
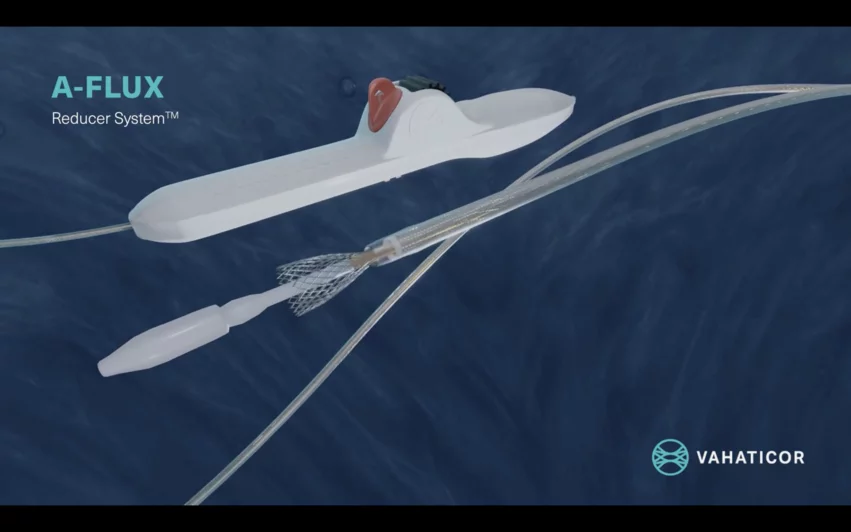
The A-Flux Reducer System was designed and developed by VahatiCor, a new medical device company associated with California-based T45 Labs. It was built with a dense, but flexible nitinol mesh and VahatiCor intends to make it available in four different sizes. The self-expandable device is implanted percutaneously into the patient’s coronary sinus, where it increases blood flow in a way that provides more oxygen to the patient’s heart and relieves lingering symptoms. It was designed to “conform seamlessly” to any patient’s anatomy, according to VahatiCor, and it can be repositioned or retrieved as necessary.
The procedure was led by interventional cardiologists Jean-Michel Paradis, MD, and Can Manh Nguyen, MD, with the Quebec Heart and Lung Institute. VahatiCor announced the successful implant in February, with CEO Howard Edelman noting that additional implants are on the horizon, and now the cardiologists have detailed their experience with the device in EuroIntervention.[1]
“The first-in-human implantation of the A-Flux coronary sinus reducer was performed in a 67-year-old male patient with a history of severe coronary artery disease, with prior quadruple coronary artery bypass graft surgery in 2000,” the authors wrote. “In 2015, he developed severe stable angina, with an angiogram revealing two occluded saphenous vein grafts to two marginals and a severe lesion in the left anterior descending artery (LAD) distal to the right mammary artery anastomosis. The left mammary graft to the first marginal was patent, providing retrograde filling to the marginals. Following percutaneous coronary intervention on the distal LAD, his symptoms improved, but angina recurred in 2023.”
A perfusion scan identified severe ischemia compromising 50% of the patient’s left ventricle, and angiography confirmed the patient now had two new LAD lesions that were both “unsuitable for revascularization.” In addition, the patient was still presenting with severe angina symptoms, even after being treated with beta-blockers, long-acting nitrates, calcium channel blockers and ranolazine.
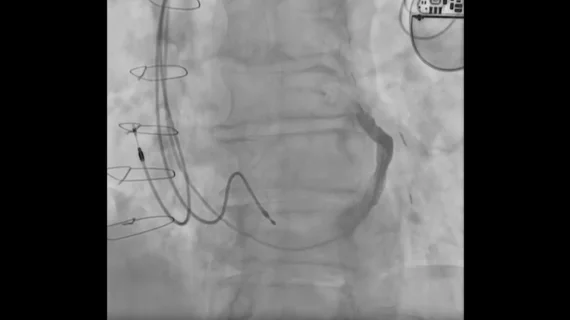
Paradis et al. implanted the device using right jugular access with the patient under conscious sedation.
“After coronary sinus injection and device sizing using fluoroscopy and intravascular ultrasound, a coronary sinus wedge pressure assessment of potential treatment responsiveness was undertaken,” the authors explained. “This is an important step, since an acute increase in coronary sinus wedge pressure leads to a decrease in microvascular resistance and an increase in blood flow. However, the pressure does not increase to the same extent in all patients. Indeed, patients with higher increases usually show better responsiveness to coronary sinus reduction therapy.”
The device was successfully implanted, and the patient presented with a “significant improvement” in quality of life after one month. Follow-up CT imaging confirmed the device was in the correct position.
The care team emphasized that patients should be prescribed 80 mg of aspirin indefinitely and 75 mg of clopidogrel for at least six months following treatment. The group also wrote that self-expanding devices do present a “theoretical risk of dislodgement,” but “controlled deployment and adequate oversizing” should address any cardiologist concerns.
“Although encouraging, these results are the first-in-human experience and, thus, are applicable only to this patient,” the authors concluded. “Large prospective sham-controlled trials are needed to determine the efficacy of this new technology.”
The full study is available in EuroIntervention, a journal from EuroPCR and the European Association of Percutaneous Cardiovascular Interventions.