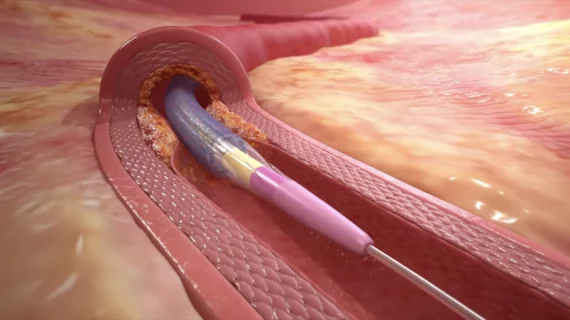
Medtronic enrolls first patient in new coronary DCB trial—data could lead to FDA approval
Medtronic has officially started enrolling patients into a new global trial focused on using the company’s Prevail paclitaxel-coated balloon catheter during percutaneous coronary intervention (PCI) to treat in-stent restenosis (ISR) and de novo small vessel disease in patients with coronary artery disease (CAD).
The Prevail drug-coated balloon (DCB) inflates within the patient’s artery during PCI, delivering paclitaxel to their arterial tissue to be absorbed and retained. This is designed to prevent ISR from recurring, keeping patients from experiencing repeat hospitalizations for the same issue. The device’s FreePac coating has already been established as an effective tool in patients with both CAD and peripheral artery disease.
Cardiologist Ziad Ali, MD, DPhil, director of the DeMatteis Cardiovascular Institute at St. Francis Hospital and Heart Center in New York, performed the first PCI for this global study.
“CAD is a chronic condition affecting over 315 million people globally, highlighting the importance of developing innovative and long-lasting solutions to assist patients in maintaining blood flow, whether they have new heart blockages or have been previously treated,” Ali said in a statement. “In the U.S., drug-coated balloons have been used for more than a decade in patients with peripheral artery disease. This trial will not only bring the use of this innovative DCB technology to patients with previously treated blockages—where stents have failed—but it will also bring use to new blockages in small vessels—where stents might not perform as well in the coronary arteries. We are proud to be enrolling patients in the Prevail Global study.”
The Prevail Global study is expected to enroll up to 1,205 CAD patients throughout the United States, Europe and Asia. It was designed to specifically investigate the Prevail DCB’s safety and effectiveness in patients with ISR and de novo small vessel disease. Target lesion failure one year after treatment is the tria’s primary endpoint.
The Prevail DCB gained CE mark approval back in 2001 and is already available in dozens of countries. Medtronic hopes patient data from this trial will help the company gain regulatory approvals in the United States and Japan.
Coronary DCBs gaining momentum in United States
DCBs have been used during PCI procedures in other parts of the world for years at this point, but approvals in the U.S. have only just now started to occur. Boston Scientific’s Agent DCB, for instance, gained full U.S. Food and Drug Administration approval in March 2024. The approval was celebrated at the time by interventional cardiologists and general cardiologists alike.