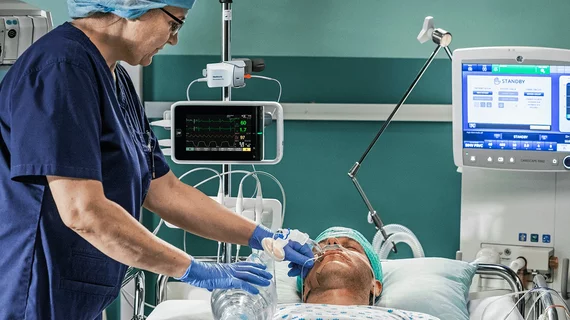
Medtronic, GE Healthcare join forces on new-look continuous monitoring solution
Medtronic and GE Healthcare have received U.S. Food and Drug Administration (FDA) clearance and European CE mark approval for a new collaboration designed to improve patient monitoring.
The combined solution involves the integration of Medtronic’s Invos regional oximetry and Microstream capnography technologies into GE Healthcare’s Carescape precision monitoring platform. It tracks the patient before, during and after key procedures, interacting with the electronic medical record (EMR).
The Invos regional oximetry technology helps track hemodynamic changes and other symptoms of cerebral desaturation events. According to Medtronic, the tool has been associated with helping patients spend much less time in the hospital following coronary artery bypass grafting procedures.
The Microstream capnography technology, meanwhile, monitors patients for signs of declining health and, according to Medtronic, can reduce insignificant alarms by more than 50%.
GE Healthcare’s FlexAcuity technology, designed to help healthcare providers personalize care as needed, is also included in this new offering.
“Medtronic and GE Healthcare are both committed to expanding access to patient care around the world,” Frank Chan, president of Medtronic’s patient monitoring business, said in a statement. “This collaboration brings together our combined medical technology leadership to improve workflow, allowing clinicians more time to focus on what matters most — the patient. Together, we are empowering clinicians with actionable insights to personalize care.”
Related Cardiac Surgery Content:
Transcatheter PVL closure linked to lower 30-day mortality than surgery
Open AAA repair linked to better long-term outcomes than EVAR
New data on cardiac damage before and after AVR suggests earlier treatment may be beneficial
Researchers awarded $1.4M to study heart transplants in children