Heart tissue heads to space for research on aging and impact of long spaceflights
Cardiac researchers at Johns Hopkins Medicine have a new cardiology trial that is simply out of this world; the group hopes to determine if certain medications can help prevent myocardial degradation during long-duration space flights to Mars and beyond. The team at Johns Hopkins Medicine and NASA worked together to launch human heart “tissue-on-a-chip” specimens to the International Space Station (ISS) on March 14. This study is part of a series of cardiac studies in space.
While the technology exists to carry humans into deep space, there are still a lot of questions about the impacts of microgravity, radiation and other factors on the human body over time outside of Earth's gravity and protective magnetic field. This project, Engineered Heart Tissues-2, is designed to monitor the tissue for changes in heart muscle cells’ mitochondria and ability to contract in low-gravity conditions. Astronauts on board during the mission will also introduce three FDA-approved medicines to the samples in efforts to prevent heart cell changes known or suspected to occur in those undertaking long-duration spaceflights.
The tissue samples were launched into space aboard SpaceX CRS-27, a resupply mission to the ISS.
“It’s possible that what we learn from these experiments in space could also inform how we treat age-related cardiac problems,” Deok-Ho Kim, PhD, professor of biomedical engineering at the Johns Hopkins University School of Medicine, said in a statement. He added that many heart cellular changes already detected in space explorers mimic changes linked to heart muscle aging in general, so the NASA experiment data also has a use back home on Earth.
How does a heart-on-a-chip work for cardiac research?
To develop the micro-engineered human heart tissue-on-a-chip, researchers begin with human induced pluripotent stem cells grown in the laboratory. Such cells are able to develop into nearly any type of cell, and are coaxed biologically to develop into beating human cardiomyocytes, the muscle cells that make hearts contract.
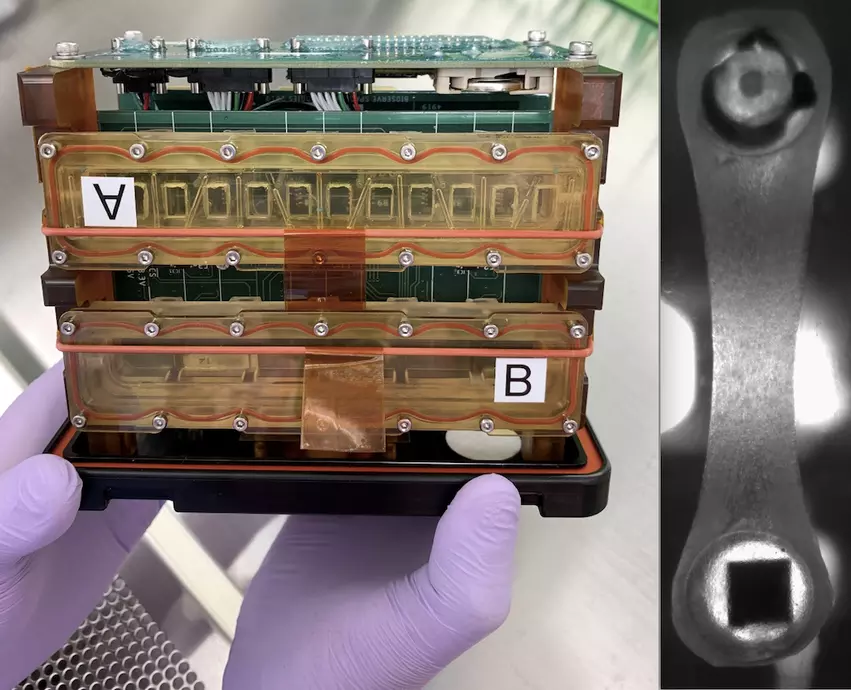
Groups of cardiomyocytes form tissue that can be strung between two posts, one flexible and one stiff. The flexible post has an embedded magnet and, when placed over sensors, allows for a collection of information on tissue contraction. The chamber enclosing the tissue is sealed so that liquid media feeding the tissue does float away in zero-gravity conditions. These tissue chambers are then loaded into so-called plate habitats with the magnetic sensors located beneath the tissue. The experimental payload consists of two of these plate habitats, which measure about 7 inches long, 5 inches tall and 4 inches wide.
This is the second Johns Hopkins cardiac experiment sent to the ISS. Kim, his former postdoctoral researcher Jonathan Tsui, and his doctoral student Devin Mair previously sent heart tissue into space in March 2020 during the first Engineered Heart Tissues study. Those experiments, presented at the Tissue Engineering and Regenerative Medicine International Society-Americas 2022 Annual Meeting, showed that microgravity in space changed the cells’ mitochondria and the tissues’ ability to contract.
Johns Hopkins Medicine researchers collaborating with NASA launched human heart “tissue-on-a-chip” specimens to the International Space Station (ISS) March 14. The cardiac myocytes will be monitored for impacts in pumping ability in the microgravity of space. Drugs will be tested o see if they improve pumping function. The right photo shows a closeup of the myocytes attached to two posts that measure contractility. Photos from Johns Hopkins
Other NASA experiments look at long spaceflight impact on the heart
Many recent medical studies on the space station use tissue chips like the Hopkins experiment. In other cases, tissues made from cells engineered to reproduce specific characteristics, and even organoid 3D structures made up of all the different types of cells in a particular organ including the heart, have been studied. These stand-ins for actual hearts enable new types of research and drug testing.
NASA said an investigation completed in 2018, Cardiac Myocytes, first showed that microgravity helps specially programmed stem cells move toward becoming new heart muscle cells. The experiment delivered frozen stem cells to the space station where crew members thawed and cultured them before returning the samples to Earth for analysis and comparison with control batches.
Subsequent research took advantage of microgravity’s effect on cell behavior and growth to create tools for further research, model disease and test potential treatments for heart damage. The MVP Cell-03 study examined the production of heart cells from human-induced pluripotent stem cells (hiPSCs) in microgravity. Pluripotent cells are cells that have started to differentiate, making them more specialized than a stem cell, but that retain the ability to become multiple cell types. MVP Cell-03 showed that microgravity increased production of cardiomyocytes from hiPSCs. This increased production could make it possible to use cultured cells to help treat spaceflight-induced cardiac abnormalities and to replenish heart cells damaged or lost due to disease on Earth. Damaged human cardiac tissues cannot repair themselves, and loss of heart cells contributes to eventual heart failure.
“If we want to use these cells for clinical applications, we need to be able to generate a lot of them in an efficient way,” said MVP Cell-03 Principal Investigator Chunhui Xu, PhD, of the Emory University School of Medicine and Children's Healthcare of Atlanta in a statement. “Heart replacement therapy, for example, requires at least a billion cardiomyocytes for just one patient.”
The research also showed that space-grown cells have appropriate structure and function. That means they can be used to test drug safety. “Now we can test in a dish whether a drug causes adverse effects,” she said. This research can even use a person’s own blood cells to produce hiPCS cells and, in turn, heart cells that can be used to determine how the individual might react to a specific drug.
The next step is to look at the quality of cells produced with the Project Eagle study, scheduled to launch later in 2023.
"What we have in our dish now is immature cells. They don’t behave the way real heart cells behave, but are more similar to embryonic heart cells," Xu explained. "Transplanting those could be an increased risk for the patient.” Project Eagle looks at whether microgravity might be an effective approach to push the cells to more mature stages.
Testing cyro-preservation of cells for space flight
Xu’s lab also tested using cryopreservation, a process of storing cells at -80°C (-112°F), as an alternative to delivering live cell cultures to the space station. The team determined that cryopreservation does not appear to negatively affect the cells and even protects them from the effects of excess gravity experienced during launch. This technique makes it easier to plan future research since experiments do not have to start as soon as the cells reach the station.
Microgravity does impact heart function
The Cardinal Heart study took place on the space station in 2021, which used engineered heart tissues to confirm that microgravity exposure causes significant changes in heart cell function and gene expression that could lead to damage. The study was a collaboration between Joseph Wu, MD, PhD, with Stanford University, and Beth Pruitt, PhD, with the University of California Santa Barbara.
The Cardinal Heart 2.0 study, which also was part of the payload in the March 14 resupply mission launch, takes this research to the next step. It uses a beating heart organoid that contains stem cell-derived cardiomyocytes, endothelial cells and cardiac fibroblasts, which form supportive connective tissue, to test whether certain drugs can reduce or prevent microgravity-induced changes. Using tissue chips to test new drugs could help reduce the need for the animal studies required before clinical trials in humans, potentially shortening the time between discovery of a drug candidate and its clinical use.
Funding for Cardinal Heart and the Engineered Heart Tissues research was provided by the National Institutes of Health (NIH).