New avenue for regenerating damaged heart cells discovered
Researchers have discovered a way to regenerate damaged heart muscle cells in mice, which may provide a new avenue for treating congenital heart defects in children, and heart attack damage in adults, according to a study published in the Journal of Clinical Investigation.[1]
Cardiomyocytes can regenerate in newborn mammals, but lose this ability with age, explained Paul Schumacker, PhD, Patrick M. Magoon Distinguished Professor in Neonatal Research at the Ann & Robert H. Lurie Children’s Hospital of Chicago, and professor of pediatrics, cell and molecular biology and medicine at Northwestern University Feinberg School of Medicine, said in a statement. Schumacker and his collaborators sought to understand if adult mammalian cardiomyocytes could revert to a regenerative fetal state.
“At the time of birth, the cardiac muscle cells still can undergo mitotic cell division,” Schumacker said. “For example, if the heart of a newborn mouse is damaged when it’s a day or two old, and then you wait until the mouse is an adult, if you look at the area of the heart that was damaged previously, you would never know that there was damage there.”
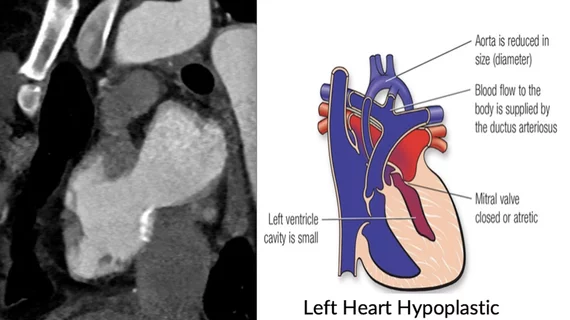
The researchers are looking for ways to treat hypoplastic left heart syndrome (HLHS), is a rare congenital heart defect that occurs when the left side of a fetal heart does not develop properly during pregnancy. The condition affects one in 5,000 newborns and is responsible for 23 percent of cardiac deaths in the first week of life, said Lurie Children’s Hospital in a statement.
Because fetal cardiomyocytes survive on glucose, instead of generating cellular energy through their mitochondria, Schumacker and his collaborators deleted the mitochondria-associated gene UQCRFS1 in the hearts of adult mice, forcing them to return to a fetal-like state.
In adult mice with damaged heart tissue, investigators observed that heart cells began regenerating once UQCRFS1 was inhibited. The cells also began to take in more glucose, similar to how fetal heart cells function, according to the study. The findings suggest that causing increased glucose utilization can also restore cell division and growth in adult heart cells and may provide a new direction for treating damaged heart cells, Schumacker explained.
He said this might be the answer to address one of the most important questions in cardiology in how to heart cells to divide again to enable hearts to repair themselves. Schumacker and his collaborators will now focus on identifying drugs that can trigger this response in heart cells without genetic manipulation.
“If we could find a drug that would turn on this response in the same way the gene manipulation did, we could then withdraw the drug once the heart cells have grown,” Schumacker added. “In the case of children with HLHS, this may allow us to restore the normal thickness to the left ventricular wall. That would be lifesaving.”
The same approach could also be used for adults who have suffered heart damage due an infarct, which could have a significant impact in reducing the incidence of heart failure.
The study was supported by National Institutes of Health grants HL35440, HL122062, HL118491 and HL109478.