CMS updates outpatient reimbursement policy for Barostim device
CVRx, a Minneapolis-based healthcare technology company, announced that baroreflex activation therapy with its implantable Barostim device has been assigned to New Technology Ambulatory Payment Classification (APC) 1580. The news, made official when the Centers for Medicare and Medicaid Services (CMS) released its final rule for the 2025 Medicare Hospital Outpatient Prospective Payment System, means Barostim procedures are associated with an APC payment of approximately $45,000 that will last at least through the end of 2025.
“We applaud this action by CMS, which appropriately recognizes the resource requirements associated with the Barostim implant procedure in the outpatient setting,” Kevin Hykes, president and CEO of CVRx, said in a statement.” We appreciate the support from the CMS Hospital Outpatient Physician Advisory Panel, medical societies and the hospital and physician community throughout the public comment period.”
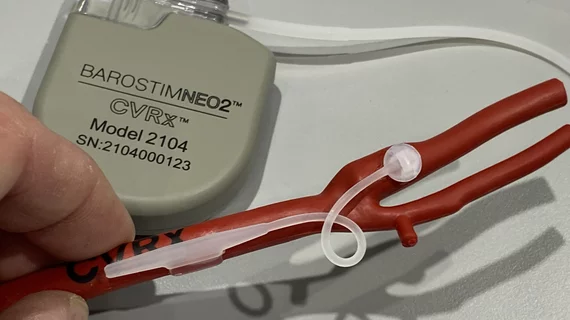
What is the Barostim implant?
Barostim is an implantable device designed for the treatment of patients with congestive heart failure and a low or reduced ejection fraction. It has gained both U.S. Food and Drug Administration and CE mark approval.
The implant works by sending small electrical pulses to certain sensors in the neck, which then send signals to the brain to help regulate the patient’s heart, kidneys and vascular system. These signals are typically sent without therapy, but this function is diminished in heart failure patients, leading to significant health risks.
More good news for CVRx
Minnesota-based CVRx has gained significant momentum as 2024 comes to a close, with Barostim being associated with one positive policy announcement after another.
In August, for example, CMS associated the Barostim device with a higher MS-DRG, increasing the inpatient payment hospitals will receive from $17,000-$23,000 to $43,000. In October, meanwhile, Barostim received new Category I CPT codes from the American Medical Association (AMA). The new codes are permanent, a sign that CMS is confident in the long-term safety and effectiveness of the Barostim procedure, and go into effect in January 2026.
This series of updates, Hykes said, “represent a fundamental and comprehensive improvement in physician coding and hospital reimbursement” for baroreflex activation therapy with Barostim.
“This will facilitate broader patient access to Barostim therapy, further strengthening our commercial foundation,” he added.
A mix of reactions for latest round of CMS policies
CMS released multiple final rules on Friday, Nov. 1, resulting in outrage from healthcare providers over some details and cheers over others. The final rule for the 2025 Medicare Physician Fee Schedule, for example, included a conversion factor reduction of 2.8% that physician groups fought to overturn for months. On the other hand, the 2025 Medicare Hospital Outpatient Prospective Payment System included a significant increase in the reimbursement payments hospitals will receive for performing coronary computed tomography angiography exams. That move has been celebrated throughout the cardiac imaging community. Read more.