New Category I CPT codes announced for treating heart failure with implantable Barostim device
CVRx, a Minneapolis-based healthcare technology company focused on heart failure treatments, announced that baroreflex activation therapy with its implantable Barostim device has received new Category I CPT codes from the American Medical Association (AMA).
“We are very pleased that the AMA’s CPT Editorial Panel approved the conversion to Category I codes,” Kevin Hykes, CEO of CVRx, said in a statement. “The Category I code designation represents an important milestone for the company and is a testament to the increased adoption, safety, and effectiveness of Barostim as an important option for patients suffering from the debilitating symptoms of heart failure. We greatly appreciate the support and guidance that Society of Vascular Surgery and American College of Cardiology provided throughout this process.”
The new codes go into effect in January 2026. AMA representatives made their decision to add the new code during a monthly panel.
For now, the new codes are known as 64XX5, 64XX6, 64XX7, 64XX8, 64XX9, 64X10, 93XX4 and 93XX5. They will be updated to full numbers closer to the time they go into effect.
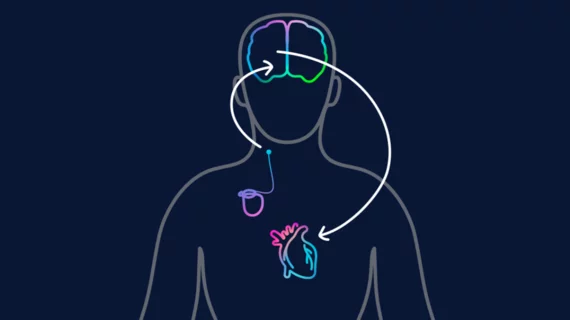
What is the Barostim implant?
Barostim is an implantable device designed for the treatment of patients with congestive heart failure and a low or reduced ejection fraction. It has gained both U.S. Food and Drug Administration and CE mark approval.
The implant works by sending small electrical pulses to certain sensors in the neck, which then send signals to the brain to help regulate the patient’s heart, kidneys and vascular system. These signals are typically sent without therapy, but this function is diminished in heart failure patients, leading to significant health risks.
Patients who already have a defibrillator are still eligible to receive a Barostim device. In fact, a majority of Barostim patients already have a defibrillator.
In August, the U.S. Centers for Medicare and Medicaid Services increased the inpatient payment associated with the Barostim device. The CMS update more than doubled the average payment amount inpatient healthcare providers receive when using baroreflex activation therapy to treat heart failure symptoms.
Register now for free webinar to learn more
Cardiovascular Business hosts a free webinar focused on the Barostim device on Thursday, Oct. 24. The webinar will include both an expert panel and a live Q&A session. Topics are expected to include how the device works, billing and reimbursement, prior authorization policies and how to increase awareness about the device among referring physicians.
Click here for more information about the upcoming webinar, including how to register.