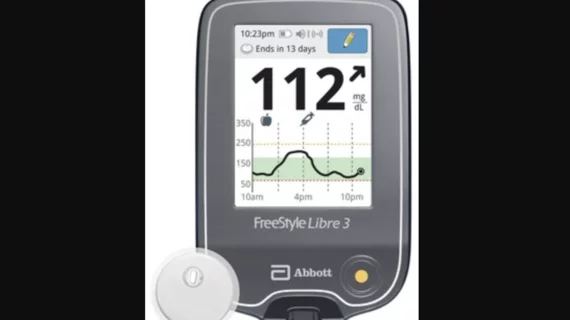
Abbott’s newest glucose monitoring device gains FDA clearance
The U.S. Food and Drug Administration (FDA) has cleared Abbott’s FreeStyle Libre 3 integrated continuous glucose monitoring (iCGM) system. The handheld device, the latest addition to Abbott’s FreeStyle Libre family of products, displays real-time glucose readings taken from a sensor worn on the user’s upper arm. It is also compliant with Abbott’s FreeStyle Libre smartphone apps, and the company has said it includes “the world’s smallest, thinnest and most discreet glucose sensor.”
“Our customers all over the world consistently tell us how our FreeStyle Libre technology has made an enormous, positive impact on their health and quality of life – they spend less time worrying and more time living,” Jared Watkin, senior vice president for Abbott's diabetes care business, said in a prepared statement. “The FreeStyle Libre 3 reader provides more choice to people living with diabetes to have access to lifesaving technology that is smaller and easier to use and comes without the high-cost burdens of other systems.”
In the same statement, Abbott noted that it is “working to get the FreeStyle Libre 3 system added to Medicare's list of covered systems as soon as possible.”
Recent issues with Abbott’s FreeStyle Libre devices
Abbott’s big news comes after the company recalled more than 4.2 million FreeStyle Libre, Libre 14 Day and Libre 2 Flash glucose management systems due to issues with their reader batteries overheating. A total of 206 incidents were reported worldwide, which covers just 0.0017% of all readers.
It was a Class I recall, meaning the FDA believed the use of those devices could cause serious injuries or death, but no request was ever made for customers to return impacted devices to the manufacturer. Instead, the FDA and Abbott both focused on reminding customers and patients that all FreeStyle Libre devices need to be properly charged and stored using only Abbott-provided cables and power adapters.
With these recent issues in mind, Abbott did emphasize in its prepared statement that it is vital to properly store, charge and use the FreeStyle Libre 3 iCGM system.