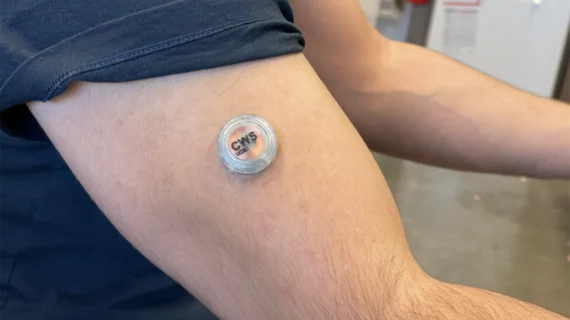
Small wearable device monitors blood sugar, alcohol and lactate levels at the same time
Engineers have developed and tested a new wearable device capable of monitoring a person’s blood sugar, alcohol and lactate levels all at once, detailing their work in Nature Biomedical Engineering.[1]
The device in question — not much larger than a small stack of quarters — is applied to the wearer’s upper arm using microneedles. The tips of the microneedles react to the person’s interstitial fluid, communicating data directly to a custom-designed smartphone app.
The team behind the device say it helps emphasize the relationship between changes in a person’s blood sugar and lifestyle choices they make related to diet, exercise and drinking alcohol.
“This is like a complete lab on the skin,” co-corresponding author Joseph Wang, DSc, a professor of nanoengineering at the University of California San Diego (UC San Diego), said in a statement. “It is capable of continuously measuring multiple biomarkers at the same time, allowing users to monitor their health and wellness as they perform their daily activities.”
“We’re starting at a really good place with this technology in terms of clinical validity and relevance,” added co-corresponding author Patrick Mercier, BSc, a professor of electrical and computer engineering at UC San Diego. “That lowers the barriers to clinical translation.”
The UC San Diego specialists did test their prototype on five volunteers thus far, asking each participant to wear the device while exercising, eating and then drinking a glass of wine. Measurements taken by the device’s microneedles were comparable to separate data collected with traditional tools.
Now, some of the device’s creators have launched a startup, AquilX, to continue developing the device.
Related Heart Health Content:
Flu shots lower CVD risk, new meta-analysis confirms
VIDEO: Use of CT to assess coronary plaques
VIDEO: USPSTF official discusses new low-dose aspirin recommendations
Statin reduces risk for non-obstructive CAD patients but no significant risk reduction for aspirin
Reference: