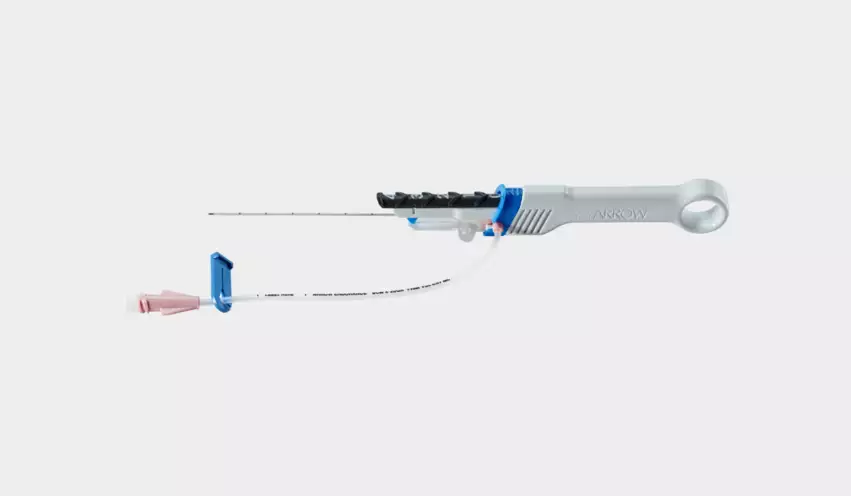
FDA announces recall of more than 250,000 interventional devices due to risk of catheter separation
The U.S. Food and Drug Administration (FDA) has announced that Arrow International, a subsidiary of Teleflex, is recalling more than 250,000 catheters after receiving reports of device separation and leakage. The FDA has said this is a Class I recall, which means using the devices “may cause serious injuries or death.”
The Arrow Endurance Extended Dwell Peripheral Catheter System is used to provide short-term access to a patient’s peripheral vascular system. This may be done while securing a blood sample, monitoring blood pressure or administering fluids.
Any catheter separation or leakage during use puts a patient at significant risks.
“If the catheter separates while in a blood vessel, the catheter fragments could be left in the bloodstream and may migrate to other places in the body,” according to an advisory on the FDA’s website. “This issue may cause serious injury, including blockage of blood vessels, inadequate blood flow, injury to blood vessel walls, blood clots, blockage of the lung arteries (pulmonary embolism), heart attack or death.”
There have been 83 complaints so far, including 18 reports of patient injuries. No deaths have been reported at this time.
The recall covers a total of 262,016 devices distributed from October 2018 to May 2023. Specific product codes included in the recall are available here.
What medical facilities and distributors should do if impacted by the FDA’s recall of the Arrow Endurance Extended Dwell Peripheral Catheter System
Medical facilities are advised to check their inventory to see if they have any products included in this recall. If they do, those products should be quarantined and not used on any patients going forward.
Customer representatives from Teleflex will reach out to each customer to help facilitate a return.
Distributors, meanwhile, are advised to immediately quarantine any affected products. They should then be returned to Teleflex.
The full FDA advisory is available here.
A recent history of Teleflex-related recalls impacting cardiologists and other cardiology professionals
Back in December, Arrow International and Teleflex recalled more than 2,000 intra-aortic balloon pumps due to significant battery issues. Teleflex also recalled a percutaneous thrombolytic device in December 2021 due to issues with its inner lumen detaching while in use.
In addition, Vascular Solutions, another subsidiary of Teleflex, recalled more than 4,000 dual lumen catheters due to separation issues in May 2020.