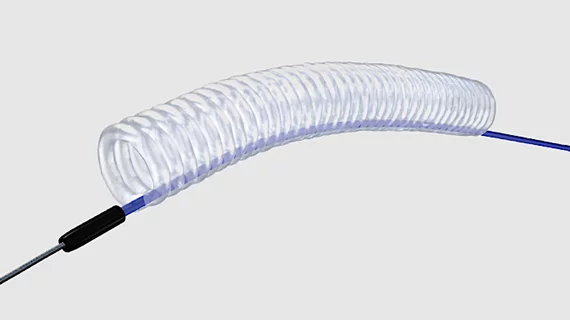
FDA clears perfusion balloon catheter for complex PCI cases
Teleflex Incorporated, a Pennsylvania-based healthcare technology company, has received U.S. Food and Drug Administration (FDA) clearance for its Ringer Perfusion Balloon Catheter (PBC). According to Teleflex, the Ringer PBC is now the only "commercially available" perfusion balloon cleared for percutaneous transluminal coronary angioplasty (PTCA), a type of percutaneous coronary intervention.
The new-look device includes a rapid-exchange catheter and a helical balloon that takes the shape of a hollow cylinder when inflated. It is indicated for balloon dilatation of the coronary artery or coronary artery bypass graft stenoses when distal blood perfusion during balloon inflation is required. In addition, the lumen inside the balloon is designed to help operators with the delivery of additional medical devices during PCI.
The FDA’s decision was in part based on promising data from the Ringer PTCA trial, a single-arm study that examined the safety and effectiveness of the Ringer PBC. The clinical trial 60 patients in the United States and Canada.
“The FDA 510(k) clearance of the Ringer PBC signifies a crucial achievement in Teleflex's commitment to advancing medical innovation and improving patient outcomes,” Christopher Buller, MD, Teleflex medical director, said in a statement. “We listen carefully to the challenges that interventionalists face daily and are proud, once again, to introduce a solution for unmet needs with this revolutionary PTCA perfusion balloon.”
“The Ringer PTCA clinical study demonstrated that inflation of the Ringer PBC for 60 seconds or more was well tolerated in the majority of patients who are vulnerable to procedural ischemia,” added Kathleen Kearney, MD, an interventional cardiologist at the University of Washington and Ringer PTCA principal investigator. “We have been eagerly awaiting the arrival of the Ringer PBC because of the potential its unique properties have to contribute to patient safety and evolve our practice in the most complex PTCA cases.”
Teleflex is aiming to begin a limited market release by August 2024.