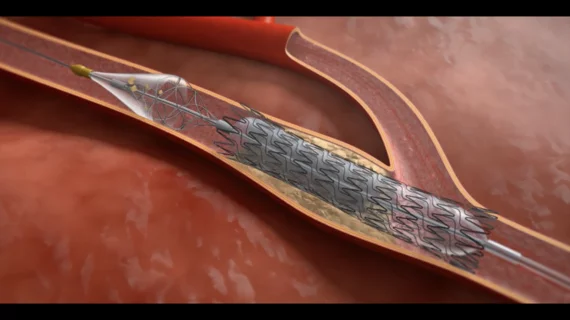
FDA gives green light to 3-in-1 carotid stent system designed to limit strokes
The U.S. Food and Drug Administration (FDA) has cleared Contego Medical’s Neuroguard IEP system for carotid arteryt stenting. The system includes a stent, integrated dilation balloon and an integrated embolic protection filter. The size of the filter can be adjusted prior to treatment based on the patient’s anatomy. In addition, it was designed with pores that are significantly smaller than traditional filters to help limit the risk of stroke or cognitive impairment.
The FDA’s decision was based on findings from the PERFORMANCE and PERFORMANCE II clinical trials designed to evaluate the Neuroguard IEP System. Data from PERFORMANCE II, for example, found that the three-in-one stenting system was associated with low stroke rates, including no major strokes, one year after treatment. Meanwhile, care teams are currently recruiting patients for PERFORMANCE III.
“FDA approval confirms the results of the clinical studies,” William Gray, MD, an interventional cardiologist with Main Line Health and co-national principle investigator of PERFORMANCE II, said in a statement. “The innovative Neuroguard IEP system performs exceptionally well with the lowest one-year stroke rates ever shown for any type of carotid revascularization, thereby establishing a new standard of care for meaningfully reducing the risk of procedural and long-term stroke among patients with carotid artery disease.”
“This FDA approval is a huge step forward for Contego and for patient care,” added Ravish Sachar, MD, Contego Medical’s CEO and founder. “The Neuroguard IEP System transforms how we approach patients with carotid artery disease by addressing the specific threats of microembolization, while simultaneously reducing procedural steps, ensuring patients receive the highest level of protection throughout the procedure.”
These devices could receive considerable attention after a 2023 policy update from the U.S. Centers for Medicare and Medicaid Services expanded coverage for percutaneous transluminal angioplasty of the carotid artery concurrent with stenting. The updated Medicare coverage is expected to increase the use of carotid artery stenting over carotid endarterectomy. To read more on that topic, read this exclusive interview with Ken Rosenfield, MD, section head of vascular medicine and intervention at Massachusetts General Hospital, from October 2023.
Watch a video interview with Rosenfield and Gray, about the impact of new CMS coverage for carotid stenting at TCT 2023.