Medtronic launches updated drug-eluting coronary stent system
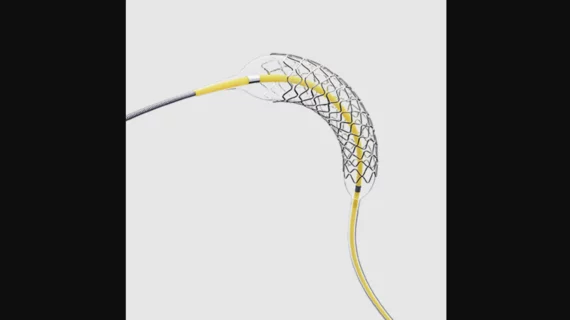
Medtronic has officially launched its Onyx Frontier drug-eluting stent (DES), a new interventional offering for coronary artery disease (CAD) designed to have an updated delivery system, improved flexibility and lower crossing profile. The company calls it “the most deliverable DES yet.”
The news comes after the Onyx Frontier DES gained CE mark approval. It received approval from the U.S. Food and Drug Administration (FDA) in May.
“The Onyx Frontier DES launch demonstrates our commitment to interventional cardiologists by providing best-in-class products,” Jason Weidman, senior vice president and president of the Medtronic’s coronary and renal denervation business unit, said in a prepared statement. “Following our launch in the U.S., we're thrilled to provide hospitals across western Europe and the globe with the Onyx Frontier DES, which has been thoughtfully designed with physicians’ needs in mind. This launch furthers Medtronic’s goal of engineering the extraordinary, and we look forward to continuing to pursue innovation each day.”
“The new Onyx Frontier DES, with its enhanced deliverability, will continue to help interventional cardiologists treat complex coronary cases and larger ranges of vessel sizes more efficiently,” Azeem Latib, MD, section head of interventional cardiology and medical director of structural heart interventions at Montefiore Medical Center in New York City, said in a previous Medtronic statement. “Delivering safe and effective outcomes to our patients is our number one priority. It's important that physicians have access to tools like the Onyx Frontier DES that can allow them to efficiently achieve those outcomes.”
This new-look solution has been approved for complex percutaneous coronary intervention (PCI), left main PCI, chronic total occlusions, the treatment of bifurcation lesions, one-month dual-antiplatelet therapy for patients facing a high risk of bleeding and other interventional treatments.
Additional information about the Onyx Frontier DES is available here. Medtronic’s detailed Onyx data is available here.