Medtronic’s more deliverable drug-eluting stent gains FDA approval
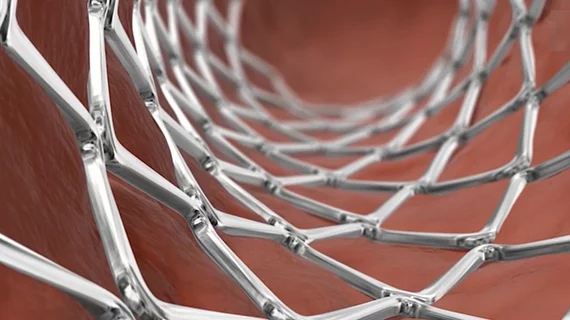
Medtronic’s Onyx Frontier drug-eluting stent (DES), offering an improved delivery system and lower crossing profile, has received U.S. Food and Drug Administration (FDA) approval.
According to Medtronic, this new solution represents “the latest evolution” of its Resolute DES series. The company emphasized that it was designed with both deliverability and performance in mind, highlighting the stent’s new more flexible catheter, new balloon design and lower crossing profile. The system uses the Resolute Onyx DES platform.
“The new Onyx Frontier DES, with its enhanced deliverability, will continue to help interventional cardiologists treat complex coronary cases and larger ranges of vessel sizes more efficiently,” Azeem Latib, MD, section head of interventional cardiology and medical director of structural heart interventions at Montefiore Medical Center in New York City, said in a statement. “Delivering safe and effective outcomes to our patients is our number one priority. It's important that physicians have access to tools like the Onyx Frontier DES that can allow them to efficiently achieve those outcomes.”
“The Onyx Frontier DES FDA approval is a very important milestone for Medtronic's Coronary business and demonstrates our commitment to interventional cardiologists by providing best-in-class products,” added Jason Weidman, senior vice president and president of Medtronic’s coronary and renal denervation business. “The Onyx Frontier launch also correlates directly to Medtronic's commitment to engineering. The team built upon the design and clinical successes of the Resolute Onyx DES and has continued to evolve proven DES technology to further address the needs of physicians.”
The Onyx Frontier is available in multiple sizes, including 4.5 mm and 5 mm, and can be expanded to 6 mm to treat patients with larger vessels.
Related Interventional Cardiology Content:
SCAI 2022 late-breaking clinical research presentations announced
Transcaval TAVR outperforms transaxillary TAVR when femoral access is not an option
VIDEO: Shockwave Medical demonstrates faster intravascular lithotripsy at ACC.22